J Pathol Transl Med.
2022 Sep;56(5):301-308. 10.4132/jptm.2022.08.03.
Papillary and medullary thyroid carcinomas coexisting in the same lobe, first suspected based on fine-needle aspiration cytology: a case report
- Affiliations
-
- 1Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2533707
- DOI: http://doi.org/10.4132/jptm.2022.08.03
Abstract
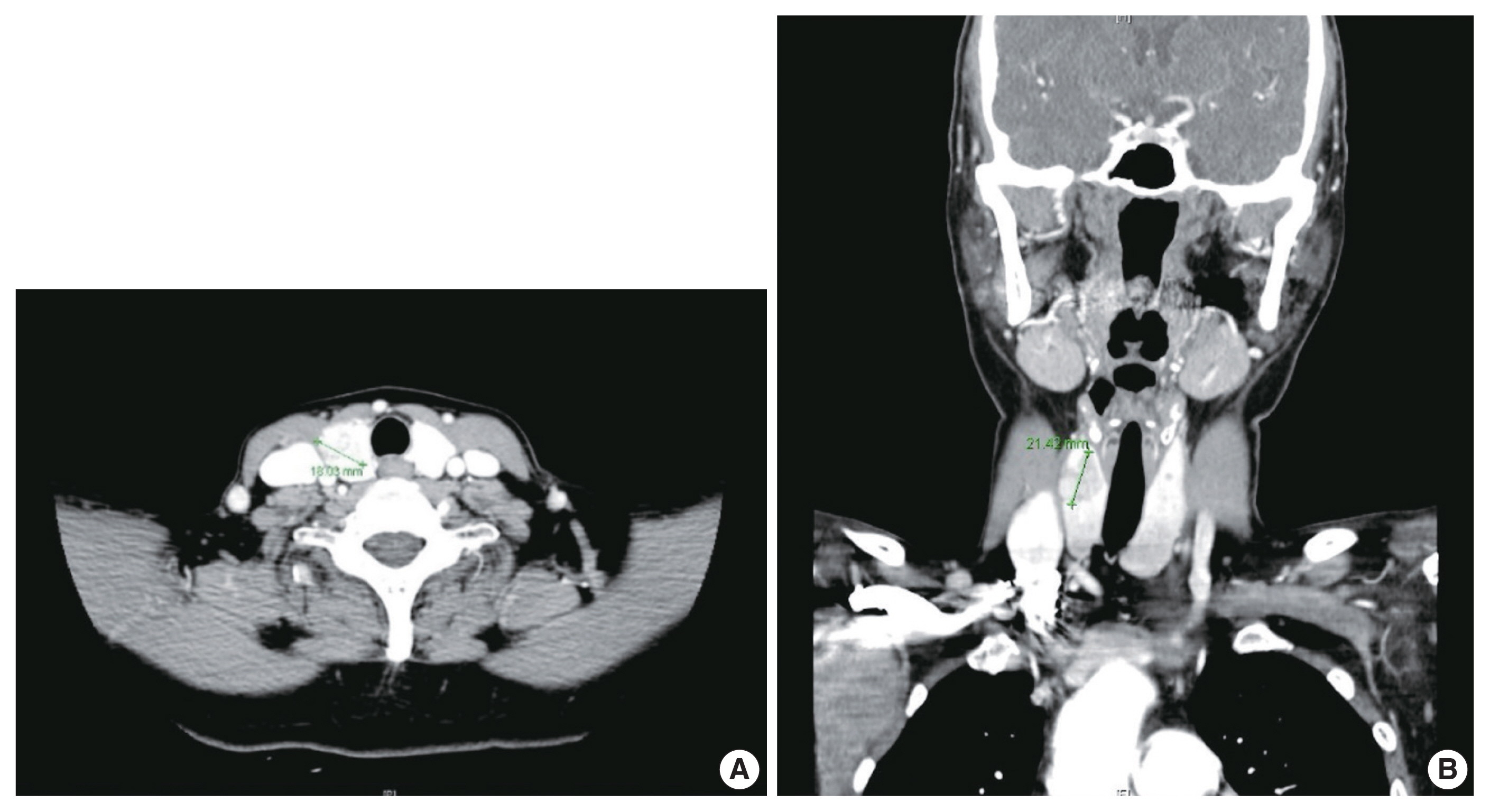
- Because different types of thyroid malignancies have distinct embryological origins, coexisting tumors are rarely observed. We describe a coexisting papillary thyroid carcinoma (PTC) and medullary thyroid carcinoma (MTC) first suspected by fine-needle aspiration cytology (FNAC). A 57-year-old female presented with an irregular mass in the right thyroid lobe. The cytopathologic findings of fine-needle aspiration showed two components: a papillary-like arrangement consisting of cells with pale enlarged nuclei indicative of PTC and loose clusters comprised of oval cells with granular chromatin indicative of MTC. The diagnosis of a coexisting PTC and MTC was initially confirmed by calcitonin immunocytochemistry and later after total thyroidectomy. Although some surgical case reports of PTC and MTC coexisting in either the same or different lobes have been documented, a case suspected by FNAC before the surgery has rarely been reported. Because appropriate treatment and prognosis of PTC and MTC are different, cytopathologists should be aware of this rare entity.
Figure
Reference
-
References
1. Kazaure HS, Roman SA, Sosa JA. Aggressive variants of papillary thyroid cancer: incidence, characteristics and predictors of survival among 43,738 patients. Ann Surg Oncol. 2012; 19:1874–80.
Article2. Sadat Alavi M, Azarpira N. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumor with lymph node metastasis: a case report. J Med Case Rep. 2011; 5:590.
Article3. De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocr Rev. 2004; 25:722–46.
Article4. Baloch ZW, LiVolsi VA. Special types of thyroid carcinoma. Histopathology. 2018; 72:40–52.
Article5. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003; 63:1454–7.6. Marsh DJ, Learoyd DL, Andrew SD, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf). 1996; 44:249–57.7. Alberti L, Carniti C, Miranda C, Roccato E, Pierotti MA. RET and NTRK1 proto-oncogenes in human diseases. J Cell Physiol. 2003; 195:168–86.
Article8. Greco C, Brigante G, Taliani E, Corrado S, Simoni M, Madeo B. Concomitant medullary thyroid carcinoma with paraganglioma-like pattern and papillary thyroid carcinoma. Endocrinol Diabetes Metab Case Rep. 2019; 2019:19–0094.
Article9. Kim WG, Gong G, Kim EY, et al. Concurrent occurrence of medullary thyroid carcinoma and papillary thyroid carcinoma in the same thyroid should be considered as coincidental. Clin Endocrinol (Oxf). 2010; 72:256–63.
Article10. Maitra A. The endocrine system. Kumar V, Abbas AK, Aster JC, editors. Robbins and Cotran pathologic basis of disease. 9th ed. Philadelphia: Elsevier publications;2015. p. 1073–140.
Article11. Braverman LE. Werner and Ingbar’s the thyroid: a fundamental and clinical text. Philadelphia: Lippincott Williams and Wilkins;2005.12. Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015; 25:567–610.
Article13. Jimenez C, Hu MI, Gagel RF. Management of medullary thyroid carcinoma. Endocrinol Metab Clin North Am. 2008; 37:481–96.
Article14. Rossi S, Fugazzola L, De Pasquale L, et al. Medullary and papillary carcinoma of the thyroid gland occurring as a collision tumour: report of three cases with molecular analysis and review of the literature. Endocr Relat Cancer. 2005; 12:281–9.
Article15. Sizemore GW. Medullary carcinoma of the thyroid gland. Semin Oncol. 1987; 14:306–14.16. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs . 4th ed. Lyon: IARC Press;2017.17. Lamberg BA, Reissel P, Stenman S, et al. Concurrent medullary and papillary thyroid carcinoma in the same thyroid lobe and in siblings. Acta Med Scand. 1981; 209:421–4.
Article18. Younes N, Shomaf M, Al Hassan L. Simultaneous medullary and papillary thyroid carcinoma with lymph node metastasis in the same patient: case report and review of the literature. Asian J Surg. 2005; 28:223–6.
Article19. Ahn D, Sohn JH, Park JY. A case of concurrent papillary and medullary thyroid carcinomas detected as recurrent medullary carcinoma after initial surgery for papillary carcinoma. J Korean Thyroid Assoc. 2013; 6:80–4.
Article20. Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology: definitions, criteria and explanatory notes. 2nd ed. Cham: Springer;2010.21. Tuttle M, Morris LF, Haugen B, et al. Thyroid-differentiated and anaplastic carcinoma. Amin MB, Edge SB, Greene F, editors. AJCC cancer staging manual. 8th ed. New York: Springer International Publishing;2017. p. 881–98.22. Cibas ES, Ali SZ. The 2017 Bethesda System for Reporting Thyroid Cytopathology. J Am Soc Cytopathol. 2017; 6:217–22.
Article23. Green I, Ali SZ, Allen EA, Zakowski MF. A spectrum of cytomorphologic variations in medullary thyroid carcinoma: fine-needle aspiration findings in 19 cases. Cancer. 1997; 81:40–4.
Article24. Trimboli P, Treglia G, Guidobaldi L, et al. Detection rate of FNA cytology in medullary thyroid carcinoma: a meta-analysis. Clin Endocrinol (Oxf). 2015; 82:280–5.
Article25. Liu CY, Chen CC, Bychkov A, et al. Constitutive cytomorphologic features of medullary thyroid carcinoma using different staining methods. Diagnostics (Basel). 2021; 11:1396.
Article26. Papaparaskeva K, Nagel H, Droese M. Cytologic diagnosis of medullary carcinoma of the thyroid gland. Diagn Cytopathol. 2000; 22:351–8.
Article27. Biscolla RP, Ugolini C, Sculli M, et al. Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid. 2004; 14:946–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Mixed Follicular-Papillary Thyroid Carcinoma
- A Case of Concurrent Medullary and Papillary Carcinoma of the Thyroid Gland
- Concurrent Papillary and Medullary Carcinoma of the Thyroid Gland
- Fine needle aspiration cytology of medullary carcinoma of the thyroid gland: a case report
- A Case of Concurrent Papillary and Medullary Thyroid Carcinomas Detected as Recurrent Medullary Carcinoma after Initial Surgery for Papillary Carcinoma