Diabetes Metab J.
2022 Sep;46(5):756-766. 10.4093/dmj.2021.0166.
Synergistic Interaction between Hyperuricemia and Abdominal Obesity as a Risk Factor for Metabolic Syndrome Components in Korean Population
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea
- 3Division of Biostatistics, Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea
- KMID: 2533644
- DOI: http://doi.org/10.4093/dmj.2021.0166
Abstract
- Background
The present study investigated the role of synergistic interaction between hyperuricemia and abdominal obesity as a risk factor for the components of metabolic syndrome.
Methods
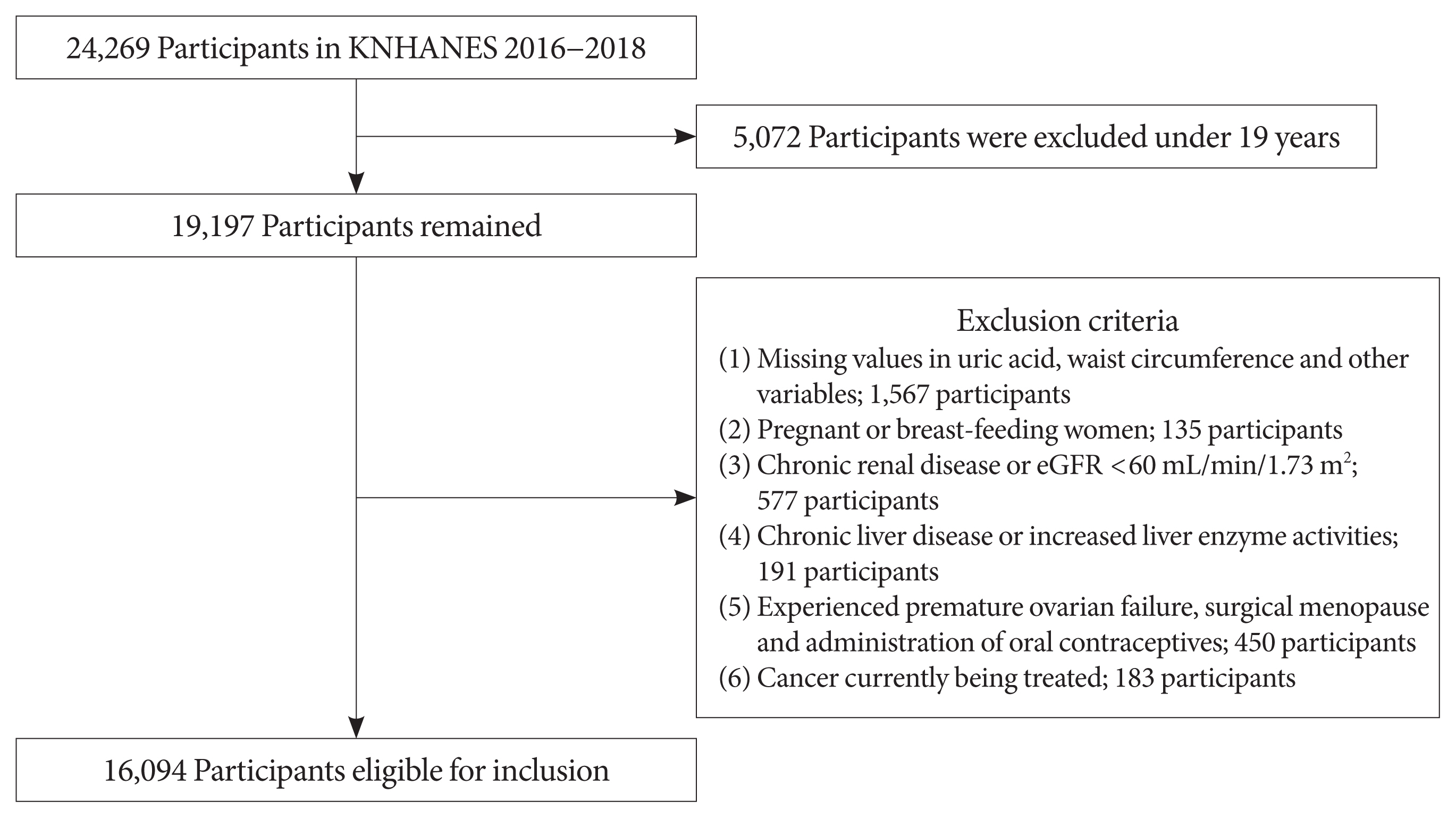
We performed a cross-sectional study using the data of 16,094 individuals from the seventh Korean National Health and Nutrition Examination Survey (2016 to 2018). The adjusted odds ratios of metabolic syndrome and its components were analyzed by multivariate logistic regression analysis. The presence of synergistic interaction between hyperuricemia and abdominal obesity was evaluated by calculating the additive scales—the relative excess risk due to interaction, attributable proportion due to interaction, and synergy index (SI).
Results
There was a synergistic interaction between hyperuricemia and abdominal obesity in hypertriglyceridemia (men: SI, 1.39; 95% confidence interval [CI], 1.01 to 1.98; women: SI, 1.61; 95% CI, 1.02 to 2.69), and low high-density lipoprotein cholesterol (HDL-C) (men: SI, 2.03; 95% CI, 1.41 to 2.91; women: SI, 1.70; 95% CI, 1.05 to 2.95). There was no significant synergistic interaction between hyperuricemia and abdominal obesity for the risk of high blood pressure (men: SI, 1.22; 95% CI, 0.85 to 1.77; women: SI, 1.53; 95% CI, 0.79 to 2.97), and hyperglycemia (men: SI, 1.03; 95% CI, 0.72 to 1.47; women: SI, 1.39; 95% CI, 0.75 to 2.57).
Conclusion
Hyperuricemia and abdominal obesity synergistically increased the risk of hypertriglyceridemia and low HDL-C in both sexes.
Keyword
Figure
Reference
-
1. Cirillo P, Sato W, Reungjui S, Heinig M, Gersch M, Sautin Y, et al. Uric acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol. 2006; 17(12 Suppl 3):S165–8.2. Kanbay M, Jensen T, Solak Y, Le M, Roncal-Jimenez C, Rivard C, et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med. 2016; 29:3–8.
Article3. Kim Y, Kang J, Kim GT. Prevalence of hyperuricemia and its associated factors in the general Korean population: an analysis of a population-based nationally representative sample. Clin Rheumatol. 2018; 37:2529–38.
Article4. Sui X, Church TS, Meriwether RA, Lobelo F, Blair SN. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008; 57:845–52.
Article5. Miao Z, Li C, Chen Y, Zhao S, Wang Y, Wang Z, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol. 2008; 35:1859–64.6. Lee SE, Han K, Kang YM, Kim SO, Cho YK, Ko KS, et al. Trends in the prevalence of metabolic syndrome and its components in South Korea: findings from the Korean National Health Insurance Service Database (2009–2013). PLoS One. 2018; 13:e0194490.
Article7. Maison P, Byrne CD, Hales CN, Day NE, Wareham NJ. Do different dimensions of the metabolic syndrome change together over time? Evidence supporting obesity as the central feature. Diabetes Care. 2001; 24:1758–63.8. Paley CA, Johnson MI. Abdominal obesity and metabolic syndrome: exercise as medicine? BMC Sports Sci Med Rehabil. 2018; 10:7.
Article9. Tsay YC, Chen CH, Pan WH. Ages at onset of 5 cardiometabolic diseases adjusting for nonsusceptibility: implications for the pathogenesis of metabolic syndrome. Am J Epidemiol. 2016; 184:366–77.
Article10. Lee YB, Jun JE, Lee SE, Ahn J, Kim G, Jee JH, et al. Utility of serum albumin for predicting incident metabolic syndrome according to hyperuricemia. Diabetes Metab J. 2018; 42:529–37.
Article11. Billiet L, Doaty S, Katz JD, Velasquez MT. Review of hyperuricemia as new marker for metabolic syndrome. ISRN Rheumatol. 2014; 2014:852954.12. Rospleszcz S, Dermyshi D, Muller-Peltzer K, Strauch K, Bamberg F, Peters A. Association of serum uric acid with visceral, subcutaneous and hepatic fat quantified by magnetic resonance imaging. Sci Rep. 2020; 10:442.
Article13. Hikita M, Ohno I, Mori Y, Ichida K, Yokose T, Hosoya T. Relationship between hyperuricemia and body fat distribution. Intern Med. 2007; 46:1353–8.
Article14. Yamada A, Sato KK, Kinuhata S, Uehara S, Endo G, Hikita Y, et al. Association of visceral fat and liver fat with hyperuricemia. Arthritis Care Res (Hoboken). 2016; 68:553–61.
Article15. Shirasawa T, Ochiai H, Yoshimoto T, Nagahama S, Watanabe A, Yoshida R, et al. Cross-sectional study of associations between normal body weight with central obesity and hyperuricemia in Japan. BMC Endocr Disord. 2020; 20:2.
Article16. Lim S, Shin H, Song JH, Kwak SH, Kang SM, Yoon JW, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011; 34:1323–8.17. Kim IY, Han KD, Kim DH, Eun Y, Cha HS, Koh EM, et al. Women with metabolic syndrome and general obesity are at a higher risk for significant hyperuricemia compared to men. J Clin Med. 2019; 8:837.
Article18. Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. Data resource profile: the Korea National Health and Nutrition Examination Survey (KNHANES). Int J Epidemiol. 2014; 43:69–77.
Article19. Roberts WC. The Friedewald-Levy-Fredrickson formula for calculating low-density lipoprotein cholesterol, the basis for lipid-lowering therapy. Am J Cardiol. 1988; 62:345–6.
Article20. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–70.
Article21. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–52.22. Yoon YS, Oh SW. Optimal waist circumference cutoff values for the diagnosis of abdominal obesity in Korean adults. Endocrinol Metab (Seoul). 2014; 29:418–26.
Article23. Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005; 20:575–9.
Article24. VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014; 3:33–72.
Article25. de Mutsert R, Jager KJ, Zoccali C, Dekker FW. The effect of joint exposures: examining the presence of interaction. Kidney Int. 2009; 75:677–81.
Article26. Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013; 15:175–81.
Article27. Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006; 290:F625–31.
Article28. Baldwin W, McRae S, Marek G, Wymer D, Pannu V, Baylis C, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011; 60:1258–69.
Article29. Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, et al. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism. 2011; 60:1259–70.
Article30. Yuan H, Yu C, Li X, Sun L, Zhu X, Zhao C, et al. Serum uric acid levels and risk of metabolic syndrome: a dose-response meta-analysis of prospective studies. J Clin Endocrinol Metab. 2015; 100:4198–207.
Article31. Jeong J, Suh YJ. Association between serum uric acid and metabolic syndrome in Koreans. J Korean Med Sci. 2019; 34:e307.
Article32. Lin SD, Tsai DH, Hsu SR. Association between serum uric acid level and components of the metabolic syndrome. J Chin Med Assoc. 2006; 69:512–6.
Article33. Zhang S, Du T, Li M, Lu H, Lin X, Yu X. Combined effect of obesity and uric acid on nonalcoholic fatty liver disease and hypertriglyceridemia. Medicine (Baltimore). 2017; 96:e6381.
Article34. Choi HK, Ford ES. Haemoglobin A1c, fasting glucose, serum C-peptide and insulin resistance in relation to serum uric acid levels: the Third National Health and Nutrition Examination Survey. Rheumatology (Oxford). 2008; 47:713–7.35. Jeong H, Moon JE, Jeon CH. Hyperuricemia is associated with an increased prevalence of metabolic syndrome in a general population and a decreased prevalence of diabetes in men. J Rheum Dis. 2020; 27:247–60.
Article36. Reaven GM. The kidney: an unwilling accomplice in syndrome X. Am J Kidney Dis. 1997; 30:928–31.
Article37. Tuomilehto J, Zimmet P, Wolf E, Taylor R, Ram P, King H. Plasma uric acid level and its association with diabetes mellitus and some biologic parameters in a biracial population of Fiji. Am J Epidemiol. 1988; 127:321–36.
Article38. Cook DG, Shaper AG, Thelle DS, Whitehead TP. Serum uric acid, serum glucose and diabetes: relationships in a population study. Postgrad Med J. 1986; 62:1001–6.
Article39. Palmer TM, Nordestgaard BG, Benn M, Tybjaerg-Hansen A, Davey Smith G, Lawlor DA, et al. Association of plasma uric acid with ischaemic heart disease and blood pressure: mendelian randomisation analysis of two large cohorts. BMJ. 2013; 347:f4262.
Article40. Lee SW, Kim HC, Nam C, Lee HY, Ahn SV, Oh YA, et al. Age-differential association between serum uric acid and incident hypertension. Hypertens Res. 2019; 42:428–437.
Article41. Tsushima Y, Nishizawa H, Tochino Y, Nakatsuji H, Sekimoto R, Nagao H, et al. Uric acid secretion from adipose tissue and its increase in obesity. J Biol Chem. 2013; 288:27138–49.
Article42. Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, et al. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism. 1998; 47:929–33.
Article43. Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007; 30:1647–52.
Article44. Han SJ, Boyko EJ, Kim SK, Fujimoto WY, Kahn SE, Leonetti DL. Association of thigh muscle mass with insulin resistance and incident type 2 diabetes mellitus in Japanese Americans. Diabetes Metab J. 2018; 42:488–95.
Article45. Cheng YJ, Gregg EW, De Rekeneire N, Williams DE, Imperatore G, Caspersen CJ, et al. Muscle-strengthening activity and its association with insulin sensitivity. Diabetes Care. 2007; 30:2264–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of hyperuricemia and its association with metabolic syndrome and cardiometabolic risk factors in Korean children and adolescents: analysis based on the 2016–2017 Korea National Health and Nutrition Examination Survey
- Prevalence of the Metabolic Syndrome and Its associated Factors among Elders in a Rural Community
- Interrelationship of Uric Acid, Gout, and Metabolic Syndrome: Focus on Hypertension, Cardiovascular Disease, and Insulin Resistance
- The Prevalence of Obesity, Abdominal Obesity and Metabolic Syndrome among Elderly in General Population
- Clinical Predictive Factors for Metabolic Syndrome in Obese Children and Adolescents