Anat Cell Biol.
2022 Sep;55(3):311-319. 10.5115/acb.22.034.
Effect of caffeine on genes expressions of developing retinas in the chick model
- Affiliations
-
- 1Department of Anatomy, Faculty of Science, Mahidol University, Bangkok, Thailand
- 2Chulabhorn International College of Medicine, Thammasat University, Rangsit Campus, Pathumthani, Thailand
- 3Department of Physical Therapy, Walailak University, Nakhon Si Thammarat, Thailand
- KMID: 2533357
- DOI: http://doi.org/10.5115/acb.22.034
Abstract
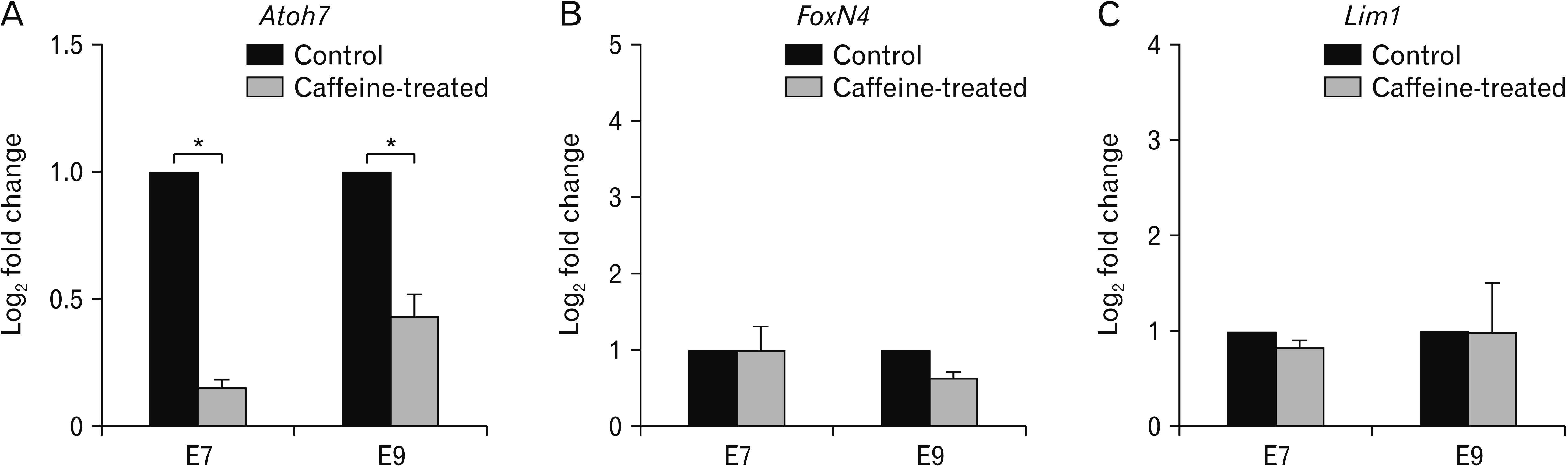
- It has been reported that overconsumption of caffeine during pregnancy leads to a deleterious effect within the nervous tissues during embryonic development. In this study, we further extrapolated the effect of caffeine in the developing retinas, which is known to be one of the most sensitive tissues in chick embryos. Morphological changes of retinal thickness and organization of neuroretinal epithelium were monitored using three gene markers, Atoh7, FoxN4, and Lim1. Upon treating with a single dose of caffeine (15 µmol at embryonic day 1 [E1]), relative thicknesses of developing retinas (particularly of E7 and E9) were significantly altered. Among the three genes studied, the expression pattern of Atoh7 was notably altered while those of FoxN4, and Lim1 mRNA showed only a slight change in these developing retinas. Quantitative polymerase chain reaction results supported the most notable changes of Atoh7 but not FoxN4, and Lim1 gene in the developing retinas, particularly at E7. The effect of caffeine towards other organs during development should be extrapolated and the awareness of its intensive consumption should be raised.
Keyword
Figure
Reference
-
References
1. Reinhardt R, Centanin L, Tavhelidse T, Inoue D, Wittbrodt B, Concordet JP, Martinez-Morales JR, Wittbrodt J. 2015; Sox2, Tlx, Gli3, and Her9 converge on Rx2 to define retinal stem cells in vivo. EMBO J. 34:1572–88. DOI: 10.15252/embj.201490706. PMID: 25908840. PMCID: PMC4474531.
Article2. Xiang M. 2013; Intrinsic control of mammalian retinogenesis. Cell Mol Life Sci. 70:2519–32. DOI: 10.1007/s00018-012-1183-2. PMID: 23064704. PMCID: PMC3566347.
Article3. Ribeiro JA, Sebastião AM. 2010; Caffeine and adenosine. J Alzheimers Dis. 20(Suppl 1):S3–15. DOI: 10.3233/JAD-2010-1379. PMID: 20164566.
Article4. Espinosa J, Rocha A, Nunes F, Costa MS, Schein V, Kazlauckas V, Kalinine E, Souza DO, Cunha RA, Porciúncula LO. 2013; Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J Alzheimers Dis. 34:509–18. DOI: 10.3233/JAD-111982. PMID: 23241554.
Article5. Madeira MH, Elvas F, Boia R, Gonçalves FQ, Cunha RA, Ambrósio AF, Santiago AR. 2015; Adenosine A2AR blockade prevents neuroinflammation-induced death of retinal ganglion cells caused by elevated pressure. J Neuroinflammation. 12:115. DOI: 10.1186/s12974-015-0333-5. PMID: 26054642. PMCID: PMC4465153.
Article6. Rana N, Moond M, Marthi A, Bapatla S, Sarvepalli T, Chatti K, Challa AK. 2010; Caffeine-induced effects on heart rate in zebrafish embryos and possible mechanisms of action: an effective system for experiments in chemical biology. Zebrafish. 7:69–81. DOI: 10.1089/zeb.2009.0631. PMID: 20415645.
Article7. Hawkins JA, Hu N, Clark EB. 1984; Effect of caffeine on cardiovascular function in the stage 24 chick embryo. Dev Pharmacol Ther. 7:334–43. DOI: 10.1159/000457182. PMID: 6478987.
Article8. Ma ZL, Qin Y, Wang G, Li XD, He RR, Chuai M, Kurihara H, Yang X. 2012; Exploring the caffeine-induced teratogenicity on neurodevelopment using early chick embryo. PLoS One. 7:e34278. DOI: 10.1371/journal.pone.0034278. PMID: 22470550. PMCID: PMC3314624. PMID: 7d0e0aa9839f42c7bccf14f64deea72e.
Article9. Park M, Choi Y, Choi H, Yim JY, Roh J. 2015; High doses of caffeine during the peripubertal period in the rat impair the growth and function of the testis. Int J Endocrinol. 2015:368475. DOI: 10.1155/2015/368475. PMID: 25983753. PMCID: PMC4423020.
Article10. Dorostghoal M, Erfani Majd N, Nooraei P. 2012; Maternal caffeine consumption has irreversible effects on reproductive parameters and fertility in male offspring rats. Clin Exp Reprod Med. 39:144–52. DOI: 10.5653/cerm.2012.39.4.144. PMID: 23346524. PMCID: PMC3548072.
Article11. Brito R, Pereira-Figueiredo D, Socodato R, Paes-de-Carvalho R, Calaza KC. 2016; Caffeine exposure alters adenosine system and neurochemical markers during retinal development. J Neurochem. 138:557–70. DOI: 10.1111/jnc.13683. PMID: 27221759.
Article12. Sheth S, Brito R, Mukherjea D, Rybak LP, Ramkumar V. 2014; Adenosine receptors: expression, function and regulation. Int J Mol Sci. 15:2024–52. DOI: 10.3390/ijms15022024. PMID: 24477263. PMCID: PMC3958836.
Article13. de Carvalho RP, Braas KM, Adler R, Snyder SH. 1992; Developmental regulation of adenosine A1 receptors, uptake sites and endogenous adenosine in the chick retina. Brain Res Dev Brain Res. 70:87–95. DOI: 10.1016/0165-3806(92)90106-7. PMID: 1473280.
Article14. Mustard JA. 2014; The buzz on caffeine in invertebrates: effects on behavior and molecular mechanisms. Cell Mol Life Sci. 71:1375–82. DOI: 10.1007/s00018-013-1497-8. PMID: 24162934. PMCID: PMC3961528.
Article15. Lee Y, Moon SJ, Montell C. 2009; Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci U S A. 106:4495–500. DOI: 10.1073/pnas.0811744106. PMID: 19246397. PMCID: PMC2657413.16. Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. 2006; A taste receptor required for the caffeine response in vivo. Curr Biol. 16:1812–7. DOI: 10.1016/j.cub.2006.07.024. PMID: 16979558.17. Nall AH, Shakhmantsir I, Cichewicz K, Birman S, Hirsh J, Sehgal A. 2016; Caffeine promotes wakefulness via dopamine signaling in Drosophila. Sci Rep. 6:20938. DOI: 10.1038/srep20938. PMID: 26868675. PMCID: PMC4751479.
Article18. Soares HC, de Melo Reis RA, De Mello FG, Ventura AL, Kurtenbach E. 2000; Differential expression of D(1A) and D(1B) dopamine receptor mRNAs in the developing avian retina. J Neurochem. 75:1071–5. DOI: 10.1046/j.1471-4159.2000.0751071.x. PMID: 10936188.
Article19. Neve KA, Seamans JK, Trantham-Davidson H. 2004; Dopamine receptor signaling. J Recept Signal Transduct Res. 24:165–205. DOI: 10.1081/RRS-200029981. PMID: 15521361.
Article20. Ma ZL, Wang G, Cheng X, Chuai M, Kurihara H, Lee KK, Yang X. 2014; Excess caffeine exposure impairs eye development during chick embryogenesis. J Cell Mol Med. 18:1134–43. DOI: 10.1111/jcmm.12260. PMID: 24636305. PMCID: PMC4508153.
Article21. Brown NL, Patel S, Brzezinski J, Glaser T. 2001; Math5 is required for retinal ganglion cell and optic nerve formation. Development. 128:2497–508. DOI: 10.1242/dev.128.13.2497. PMID: 11493566. PMCID: PMC1480839.22. Yang Z, Ding K, Pan L, Deng M, Gan L. 2003; Math5 determines the competence state of retinal ganglion cell progenitors. Dev Biol. 264:240–54. DOI: 10.1016/j.ydbio.2003.08.005. PMID: 14623245.
Article23. Le TT, Wroblewski E, Patel S, Riesenberg AN, Brown NL. 2006; Math5 is required for both early retinal neuron differentiation and cell cycle progression. Dev Biol. 295:764–78. DOI: 10.1016/j.ydbio.2006.03.055. PMID: 16690048.
Article24. Boije H, Edqvist PH, Hallböök F. 2008; Temporal and spatial expression of transcription factors FoxN4, Ptf1a, Prox1, Isl1 and Lim1 mRNA in the developing chick retina. Gene Expr Patterns. 8:117–23. DOI: 10.1016/j.modgep.2007.09.004. PMID: 18006384.
Article25. Edqvist PH. 2006. Neuronal development in the embryonic retina: focus on the characterization, generation and development of horizontal cell subtypes [PhD dissertation]. Acta Universitatis Upsaliensis;Uppsala:26. Gouge A, Holt J, Hardy AP, Sowden JC, Smith HK. 2001; Foxn4--a new member of the forkhead gene family is expressed in the retina. Mech Dev. 107:203–6. DOI: 10.1016/S0925-4773(01)00465-8. PMID: 11520680.27. Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. 2004; Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 43:795–807. DOI: 10.1016/j.neuron.2004.08.041. PMID: 15363391.
Article28. Dyer MA, Livesey FJ, Cepko CL, Oliver G. 2003; Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 34:53–8. DOI: 10.1038/ng1144. PMID: 12692551.
Article29. Marillat V, Sabatier C, Failli V, Matsunaga E, Sotelo C, Tessier-Lavigne M, Chédotal A. 2004; The slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron. 43:69–79. DOI: 10.1016/j.neuron.2004.06.018. PMID: 15233918.
Article30. Klebanoff MA, Levine RJ, Clemens JD, Wilkins DG. 2002; Maternal serum caffeine metabolites and small-for-gestational age birth. Am J Epidemiol. 155:32–7. DOI: 10.1093/aje/155.1.32. PMID: 11772782.
Article31. Bracken MB, Triche EW, Belanger K, Hellenbrand K, Leaderer BP. 2003; Association of maternal caffeine consumption with decrements in fetal growth. Am J Epidemiol. 157:456–66. DOI: 10.1093/aje/kwf220. PMID: 12615610.
Article32. Bakker R, Steegers EA, Obradov A, Raat H, Hofman A, Jaddoe VW. 2010; Maternal caffeine intake from coffee and tea, fetal growth, and the risks of adverse birth outcomes: the generation R study. Am J Clin Nutr. 91:1691–8. DOI: 10.3945/ajcn.2009.28792. PMID: 20427730.
Article33. Momoi N, Tinney JP, Liu LJ, Elshershari H, Hoffmann PJ, Ralphe JC, Keller BB, Tobita K. 2008; Modest maternal caffeine exposure affects developing embryonic cardiovascular function and growth. Am J Physiol Heart Circ Physiol. 294:H2248–56. DOI: 10.1152/ajpheart.91469.2007. PMID: 18359892.
Article34. Blazynski C, Perez MT. 1991; Adenosine in vertebrate retina: localization, receptor characterization, and function. Cell Mol Neurobiol. 11:463–84. DOI: 10.1007/BF00734810. PMID: 1683815.
Article35. Braas KM, Zarbin MA, Snyder SH. 1987; Endogenous adenosine and adenosine receptors localized to ganglion cells of the retina. Proc Natl Acad Sci U S A. 84:3906–10. DOI: 10.1073/pnas.84.11.3906. PMID: 3473489. PMCID: PMC304985.
Article36. Kvanta A, Seregard S, Sejersen S, Kull B, Fredholm BB. 1997; Localization of adenosine receptor messenger RNAs in the rat eye. Exp Eye Res. 65:595–602. DOI: 10.1006/exer.1996.0352. PMID: 9367639.
Article37. Nguyen-Ba-Charvet KT, Rebsam A. 2020; Neurogenesis and specification of retinal ganglion cells. Int J Mol Sci. 21:451. DOI: 10.3390/ijms21020451. PMID: 31936811. PMCID: PMC7014133.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Caffeine-induced endothelial cell death and the inhibition of angiogenesis
- Effect of caffeine on the adeno-type 12 virus and its antigen induction in HeLa cells and L cells
- Expression of Heat Shock Protein 27 and Alpha B Crystallin in the Retina and Optic Nerve of the Chick Embryo
- Study for the effects of the nicotine in the organ growth and histological structure of the developing chick embryo
- The Effect of Ca++ on Neurulation of Early Chick Embryos