Korean J Physiol Pharmacol.
2022 Sep;26(5):307-312. 10.4196/kjpp.2022.26.5.307.
Distinct cell populations of ventral tegmental area process motivated behavior
- Affiliations
-
- 1School of Biological Sciences, Seoul National University, Seoul 08826, Korea
- KMID: 2532760
- DOI: http://doi.org/10.4196/kjpp.2022.26.5.307
Abstract
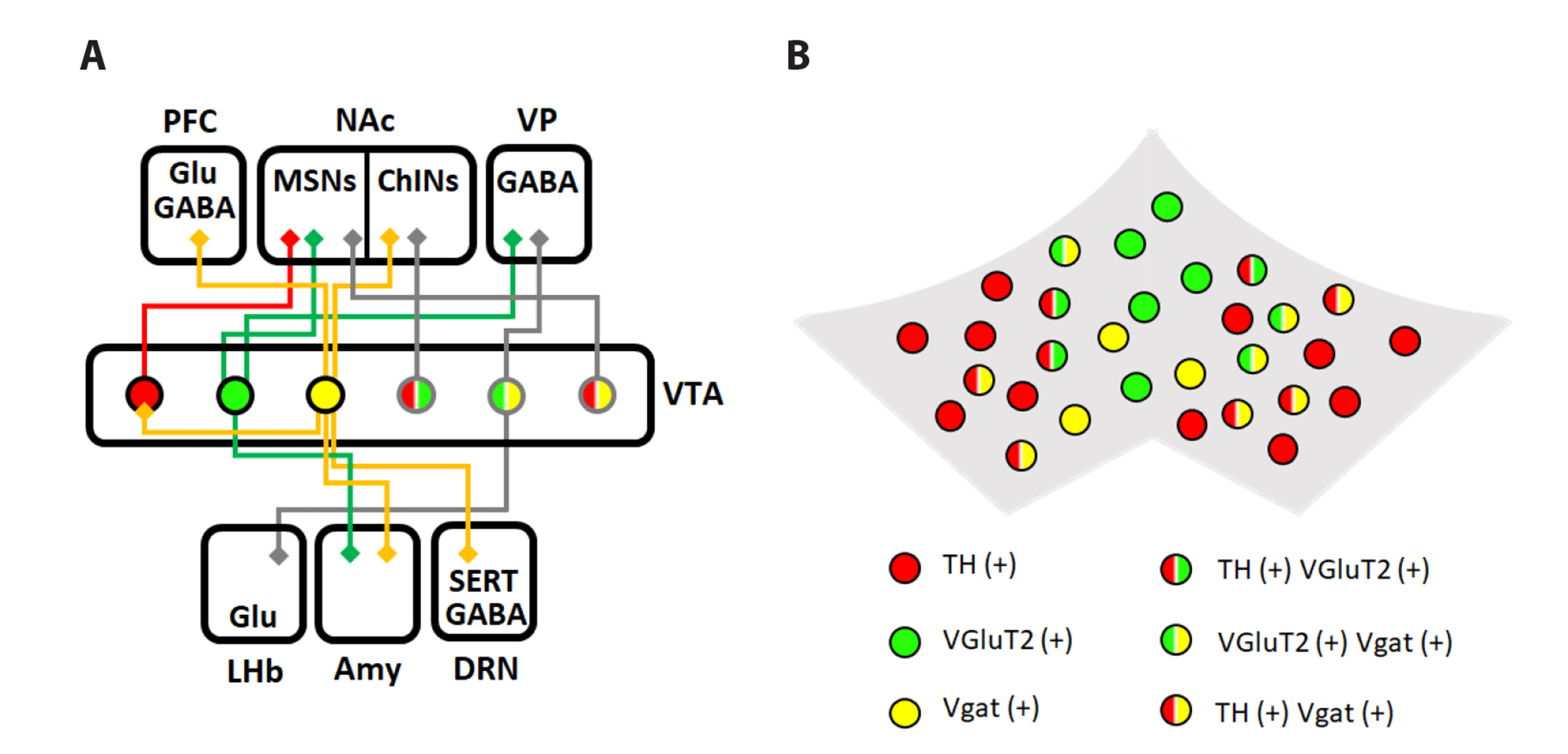
- It is well known that dopamine transmission from the ventral tegmental area (VTA) modulates motivated behavior and reinforcement learning. Although dopaminergic neurons are the major type of VTA neurons, recent studies show that a significant proportion of the VTA contains GABAergic and type 2 vesicular glutamate transporter (VGLUT2)-positive neurons. The non-dopaminergic neurons are also critically involved in regulating motivated behaviors. Some VTA neurons appear to co-release two different types of neurotransmitters. They are VGLUT2-DA neurons, VGLUT2-GABA neurons and GABA-DA neurons. These co-releasing neurons show distinct features compared to the neurons that release a single neurotransmitter. Here, we review how VTA cell populations wire to the other brain regions and how these projections differentially contribute to motivated behavior through the distinct molecular mechanism. We summarize the activities, projections and functions of VTA neurons concerning motivated behavior. This review article discriminates VTA cell populations related to the motivated behavior based on the neurotransmitters they release and extends the classical view of the dopamine-mediated reward system.
Keyword
Figure
Reference
-
1. Quessy F, Bittar T, Blanchette LJ, Lévesque M, Labonté B. 2021; Stress-induced alterations of mesocortical and mesolimbic dopaminergic pathways. Sci Rep. 11:11000. DOI: 10.1038/s41598-021-90521-y. PMID: 34040100. PMCID: PMC8154906. PMID: 25951ee047ed4c5a885a1dc34c91cdb1.
Article2. Bourdy R, Sánchez-Catalán MJ, Kaufling J, Balcita-Pedicino JJ, Freund-Mercier MJ, Veinante P, Sesack SR, Georges F, Barrot M. 2014; Control of the nigrostriatal dopamine neuron activity and motor function by the tail of the ventral tegmental area. Neuropsychopharmacology. 39:2788–2798. DOI: 10.1038/npp.2014.129. PMID: 24896615. PMCID: PMC4200489.
Article3. Stagkourakis S, Dunevall J, Taleat Z, Ewing AG, Broberger C. 2019; Dopamine release dynamics in the tuberoinfundibular dopamine system. J Neurosci. 39:4009–4022. DOI: 10.1523/JNEUROSCI.2339-18.2019. PMID: 30782976. PMCID: PMC6529860.
Article4. Spanagel R, Weiss F. 1999; The dopamine hypothesis of reward: past and current status. Trends Neurosci. 22:521–527. DOI: 10.1016/S0166-2236(99)01447-2. PMID: 10529820.
Article5. Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. 2012; Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci. 32:16223–16232. DOI: 10.1523/JNEUROSCI.3080-12.2012. PMID: 23152606. PMCID: PMC6794026.6. Park J, Lim CS, Seo H, Park CA, Zhuo M, Kaang BK, Lee K. 2015; Pain perception in acute model mice of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mol Pain. 11:28. DOI: 10.1186/s12990-015-0026-1. PMID: 25981600. PMCID: PMC4448854.7. Heinz A, Schlagenhauf F. 2010; Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 36:472–485. DOI: 10.1093/schbul/sbq031. PMID: 20453041. PMCID: PMC2879696.
Article8. Zhang JC, Lau PM, Bi GQ. 2009; Gain in sensitivity and loss in temporal contrast of STDP by dopaminergic modulation at hippocampal synapses. Proc Natl Acad Sci U S A. 106:13028–13033. DOI: 10.1073/pnas.0900546106. PMID: 19620735. PMCID: PMC2713390.
Article9. Schultz W, Apicella P, Ljungberg T. 1993; Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 13:900–913. DOI: 10.1523/JNEUROSCI.13-03-00900.1993. PMID: 8441015. PMCID: PMC6576600.
Article10. Aosaki T, Graybiel AM, Kimura M. 1994; Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 265:412–415. DOI: 10.1126/science.8023166. PMID: 8023166.
Article11. Mirenowicz J, Schultz W. 1996; Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature. 379:449–451. DOI: 10.1038/379449a0. PMID: 8559249.
Article12. Glimcher PW. 2011; Understanding dopamine and reinforcement learning: the dopamine reward prediction error hypothesis. Proc Natl Acad Sci U S A. 108(Suppl 3):15647–15654. Erratum in: Proc Natl Acad Sci U S A. 2011;108:17568-17569. DOI: 10.1073/pnas.1014269108. PMID: 21389268. PMCID: PMC3176615.
Article13. Morita K, Kato A. 2014; Striatal dopamine ramping may indicate flexible reinforcement learning with forgetting in the cortico-basal ganglia circuits. Front Neural Circuits. 8:36. Erratum in: Front Neural Circuits. 2014;8:48. DOI: 10.3389/fncir.2014.00036. PMID: 24782717. PMCID: PMC3988379.
Article14. Mohebi A, Pettibone JR, Hamid AA, Wong JT, Vinson LT, Patriarchi T, Tian L, Kennedy RT, Berke JD. 2019; Dissociable dopamine dynamics for learning and motivation. Nature. 570:65–70. Erratum in: Nature. 2019;571:E3. DOI: 10.1038/s41586-019-1235-y. PMID: 31118513. PMCID: PMC6555489.
Article15. Bromberg-Martin ES, Matsumoto M, Hikosaka O. 2010; Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 68:815–834. DOI: 10.1016/j.neuron.2010.11.022. PMID: 21144997. PMCID: PMC3032992.
Article16. Schultz W. 1998; Predictive reward signal of dopamine neurons. J Neurophysiol. 80:1–27. DOI: 10.1152/jn.1998.80.1.1. PMID: 9658025.17. Bayer HM, Glimcher PW. 2005; Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron. 47:129–141. DOI: 10.1016/j.neuron.2005.05.020. PMID: 15996553. PMCID: PMC1564381.
Article18. Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. 2012; Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 482:85–88. DOI: 10.1038/nature10754. PMID: 22258508. PMCID: PMC3271183.
Article19. Matsumoto M, Hikosaka O. 2009; Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 459:837–841. DOI: 10.1038/nature08028. PMID: 19448610. PMCID: PMC2739096.
Article20. Grace AA. 1991; Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 41:1–24. DOI: 10.1016/0306-4522(91)90196-U. PMID: 1676137.
Article21. Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. 2010; Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 30:14273–14283. DOI: 10.1523/JNEUROSCI.1894-10.2010. PMID: 20962248. PMCID: PMC6634758.
Article22. Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L. 2015; Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell. 162:622–634. DOI: 10.1016/j.cell.2015.07.015. PMID: 26232228. PMCID: PMC4522312.
Article23. Soares-Cunha C, de Vasconcelos NAP, Coimbra B, Domingues AV, Silva JM, Loureiro-Campos E, Gaspar R, Sotiropoulos I, Sousa N, Rodrigues AJ. 2020; Nucleus accumbens medium spiny neurons subtypes signal both reward and aversion. Mol Psychiatry. 25:3241–3255. Erratum in: Mol Psychiatry. 2020;25:3448. DOI: 10.1038/s41380-019-0484-3. PMID: 31462765. PMCID: PMC7714688.
Article24. Kwak S, Jung MW. 2019; Distinct roles of striatal direct and indirect pathways in value-based decision making. Elife. 8:e46050. DOI: 10.7554/eLife.46050. PMID: 31310237. PMCID: PMC6658164. PMID: 6460bd48f429464db7069fc206048432.
Article25. Sato D, Narita M, Hamada Y, Mori T, Tanaka K, Tamura H, Yamanaka A, Matsui R, Watanabe D, Suda Y, Senba E, Watanabe M, Navratilova E, Porreca F, Kuzumaki N, Narita M. 2022; Relief of neuropathic pain by cell-specific manipulation of nucleus accumbens dopamine D1- and D2-receptor-expressing neurons. Mol Brain. 15:10. DOI: 10.1186/s13041-021-00896-2. PMID: 34991655. PMCID: PMC8740378. PMID: 19e7e40f2ad442088b88dd6d70e75c50.
Article26. Cole SL, Chandra R, Harris M, Patel I, Wang T, Kim H, Jensen L, Russo SJ, Turecki G, Gancarz-Kausch AM, Dietz DM, Lobo MK. 2021; Cocaine-induced neuron subtype mitochondrial dynamics through Egr3 transcriptional regulation. Mol Brain. 14:101. DOI: 10.1186/s13041-021-00800-y. PMID: 34187517. PMCID: PMC8240292. PMID: 9d6dd2987a8d4db7bdc5e26bc0388cab.
Article27. Flanigan M, LeClair K. 2017; Shared motivational functions of ventral striatum D1 and D2 medium spiny neurons. J Neurosci. 37:6177–6179. DOI: 10.1523/JNEUROSCI.0882-17.2017. PMID: 28659329. PMCID: PMC5490058.
Article28. Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. 2015; Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci. 18:1230–1232. DOI: 10.1038/nn.4068. PMID: 26214370. PMCID: PMC4551610.
Article29. Gentry RN, Schuweiler DR, Roesch MR. 2019; Dopamine signals related to appetitive and aversive events in paradigms that manipulate reward and avoidability. Brain Res. 1713:80–90. DOI: 10.1016/j.brainres.2018.10.008. PMID: 30300635. PMCID: PMC6826219.
Article30. Kupchik YM, Kalivas PW. 2017; The direct and indirect pathways of the nucleus accumbens are not what you think. Neuropsychopharmacology. 42:369–370. DOI: 10.1038/npp.2016.160. PMID: 27909323. PMCID: PMC5143491.
Article31. Cole SL, Robinson MJF, Berridge KC. 2018; Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence. PLoS One. 13:e0207694. DOI: 10.1371/journal.pone.0207694. PMID: 30496206. PMCID: PMC6264872. PMID: 989b2923ebc9433abd1eeb8721d9327b.32. Swapna I, Bondy B, Morikawa H. 2016; Differential dopamine regulation of Ca2+ signaling and its timing dependence in the nucleus accumbens. Cell Rep. 15:563–573. DOI: 10.1016/j.celrep.2016.03.055. PMID: 27068462. PMCID: PMC4838497.
Article33. Nishi A, Kuroiwa M, Shuto T. 2011; Mechanisms for the modulation of dopamine D1 receptor signaling in striatal neurons. Front Neuroanat. 5:43. DOI: 10.3389/fnana.2011.00043. PMID: 21811441. PMCID: PMC3140648.
Article34. Zhang X, Nagai T, Ahammad RU, Kuroda K, Nakamuta S, Nakano T, Yukinawa N, Funahashi Y, Yamahashi Y, Amano M, Yoshimoto J, Yamada K, Kaibuchi K. 2019; Balance between dopamine and adenosine signals regulates the PKA/Rap1 pathway in striatal medium spiny neurons. Neurochem Int. 122:8–18. DOI: 10.1016/j.neuint.2018.10.008. PMID: 30336179.
Article35. Pupe S, Wallén-Mackenzie Å. 2015; Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA. Trends Neurosci. 38:375–386. DOI: 10.1016/j.tins.2015.04.005. PMID: 25962754.
Article36. Bariselli S, Tzanoulinou S, Glangetas C, Prévost-Solié C, Pucci L, Viguié J, Bezzi P, O'Connor EC, Georges F, Lüscher C, Bellone C. 2016; SHANK3 controls maturation of social reward circuits in the VTA. Nat Neurosci. 19:926–934. DOI: 10.1038/nn.4319. PMID: 27273769. PMCID: PMC4948673.
Article37. Poulin JF, Caronia G, Hofer C, Cui Q, Helm B, Ramakrishnan C, Chan CS, Dombeck DA, Deisseroth K, Awatramani R. 2018; Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci. 21:1260–1271. DOI: 10.1038/s41593-018-0203-4. PMID: 30104732. PMCID: PMC6342021.
Article38. Mingote S, Amsellem A, Kempf A, Rayport S, Chuhma N. 2019; Dopamine-glutamate neuron projections to the nucleus accumbens medial shell and behavioral switching. Neurochem Int. 129:104482. DOI: 10.1016/j.neuint.2019.104482. PMID: 31170424. PMCID: PMC6855309.
Article39. Zell V, Steinkellner T, Hollon NG, Warlow SM, Souter E, Faget L, Hunker AC, Jin X, Zweifel LS, Hnasko TS. 2020; VTA glutamate neuron activity drives positive reinforcement absent dopamine co-release. Neuron. 107:864–873.e4. DOI: 10.1016/j.neuron.2020.06.011. PMID: 32610039. PMCID: PMC7780844.
Article40. Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Grevès M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallén-Mackenzie A. 2010; VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A. 107:389–394. DOI: 10.1073/pnas.0910986107. PMID: 20018672. PMCID: PMC2806710.
Article41. Alsiö J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, Trudeau LÉ, Kullander K, Lévesque D, Wallén-Mackenzie A. 2011; Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J Neurosci. 31:12593–12603. DOI: 10.1523/JNEUROSCI.2397-11.2011. PMID: 21880920. PMCID: PMC6703278.
Article42. Yoo JH, Zell V, Gutierrez-Reed N, Wu J, Ressler R, Shenasa MA, Johnson AB, Fife KH, Faget L, Hnasko TS. 2016; Ventral tegmental area glutamate neurons co-release GABA and promote positive reinforcement. Nat Commun. 7:13697. DOI: 10.1038/ncomms13697. PMID: 27976722. PMCID: PMC5171775. PMID: ebc4106e19444eaba72aa7e6cdab8f51.
Article43. Salery M, Trifilieff P, Caboche J, Vanhoutte P. 2020; From signaling molecules to circuits and behaviors: cell-type-specific adaptations to psychostimulant exposure in the striatum. Biol Psychiatry. 87:944–953. DOI: 10.1016/j.biopsych.2019.11.001. PMID: 31928716.
Article44. Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. 2012; Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 32:15076–15085. DOI: 10.1523/JNEUROSCI.3128-12.2012. PMID: 23100428. PMCID: PMC3685320.
Article45. Kouwenhoven WM, Fortin G, Penttinen AM, Florence C, Delignat-Lavaud B, Bourque MJ, Trimbuch T, Luppi MP, Salvail-Lacoste A, Legault P, Poulin JF, Rosenmund C, Awatramani R, Trudeau LÉ. 2020; VGluT2 expression in dopamine neurons contributes to postlesional striatal reinnervation. J Neurosci. 40:8262–8275. DOI: 10.1523/JNEUROSCI.0823-20.2020. PMID: 32928885. PMCID: PMC7577590.
Article46. Fortin GM, Bourque MJ, Mendez JA, Leo D, Nordenankar K, Birgner C, Arvidsson E, Rymar VV, Bérubé-Carrière N, Claveau AM, Descarries L, Sadikot AF, Wallén-Mackenzie Å, Trudeau LÉ. 2012; Glutamate corelease promotes growth and survival of midbrain dopamine neurons. J Neurosci. 32:17477–17491. DOI: 10.1523/JNEUROSCI.1939-12.2012. PMID: 23197738. PMCID: PMC6621856.
Article47. Miranda-Barrientos J, Chambers I, Mongia S, Liu B, Wang HL, Mateo-Semidey GE, Margolis EB, Zhang S, Morales M. 2021; Ventral tegmental area GABA, glutamate, and glutamate-GABA neurons are heterogeneous in their electrophysiological and pharmacological properties. Eur J Neurosci. 54:4061–4084. DOI: 10.1111/ejn.15156. PMID: 33619763. PMCID: PMC8380271.
Article48. Root DH, Barker DJ, Estrin DJ, Miranda-Barrientos JA, Liu B, Zhang S, Wang HL, Vautier F, Ramakrishnan C, Kim YS, Fenno L, Deisseroth K, Morales M. 2020; Distinct signaling by ventral tegmental area glutamate, GABA, and combinatorial glutamate-GABA neurons in motivated behavior. Cell Rep. 32:108094. DOI: 10.1016/j.celrep.2020.108094. PMID: 32877676. PMCID: PMC7556367.
Article49. Bouarab C, Thompson B, Polter AM. 2019; VTA GABA neurons at the interface of stress and reward. Front Neural Circuits. 13:78. DOI: 10.3389/fncir.2019.00078. PMID: 31866835. PMCID: PMC6906177. PMID: 85dd5df8bfea41e08a65a841510005eb.
Article50. Sigel E, Steinmann ME. 2012; Structure, function, and modulation of GABAA receptors. J Biol Chem. 287:40224–40231. DOI: 10.1074/jbc.R112.386664. PMID: 23038269. PMCID: PMC3504738.51. Benarroch EE. 2012; GABAB receptors: structure, functions, and clinical implications. Neurology. 78:578–584. DOI: 10.1212/WNL.0b013e318247cd03. PMID: 22351795.
Article52. van Zessen R, Phillips JL, Budygin EA, Stuber GD. 2012; Activation of VTA GABA neurons disrupts reward consumption. Neuron. 73:1184–1194. DOI: 10.1016/j.neuron.2012.02.016. PMID: 22445345. PMCID: PMC3314244.
Article53. Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. 2012; GABA neurons of the VTA drive conditioned place aversion. Neuron. 73:1173–1183. DOI: 10.1016/j.neuron.2012.02.015. PMID: 22445344. PMCID: PMC6690362.
Article54. Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. 1994; Responses of tonically active neurons in the primate's striatum undergo systematic changes during behavioral sensorimotor conditioning. J Neurosci. 14:3969–3984. DOI: 10.1523/JNEUROSCI.14-06-03969.1994. PMID: 8207500. PMCID: PMC6576948.
Article55. Pan WX, Brown J, Dudman JT. 2013; Neural signals of extinction in the inhibitory microcircuit of the ventral midbrain. Nat Neurosci. 16:71–78. DOI: 10.1038/nn.3283. PMID: 23222913. PMCID: PMC3563090.
Article56. Eshel N, Bukwich M, Rao V, Hemmelder V, Tian J, Uchida N. 2015; Arithmetic and local circuitry underlying dopamine prediction errors. Nature. 525:243–246. Erratum in: Nature. 2015;527:398. DOI: 10.1038/nature14855. PMID: 26322583. PMCID: PMC4567485.
Article57. Wakabayashi KT, Feja M, Baindur AN, Bruno MJ, Bhimani RV, Park J, Hausknecht K, Shen RY, Haj-Dahmane S, Bass CE. 2019; Chemogenetic activation of ventral tegmental area GABA neurons, but not mesoaccumbal GABA terminals, disrupts responding to reward-predictive cues. Neuropsychopharmacology. 44:372–380. DOI: 10.1038/s41386-018-0097-6. PMID: 29875446. PMCID: PMC6300533.
Article58. Li Y, Li CY, Xi W, Jin S, Wu ZH, Jiang P, Dong P, He XB, Xu FQ, Duan S, Zhou YD, Li XM. 2019; Rostral and caudal ventral tegmental area GABAergic inputs to different dorsal raphe neurons participate in opioid dependence. Neuron. 101:748–761.e5. Erratum in: Neuron. 2021;109:3893-3894. DOI: 10.1016/j.neuron.2018.12.012. PMID: 30638902.
Article59. Tritsch NX, Oh WJ, Gu C, Sabatini BL. 2014; Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis. Elife. 3:e01936. DOI: 10.7554/eLife.01936. PMID: 24843012. PMCID: PMC4001323. PMID: 186232619a6b452a859d2b870a7b50b9.
Article60. Kim JI, Ganesan S, Luo SX, Wu YW, Park E, Huang EJ, Chen L, Ding JB. 2015; Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 350:102–106. DOI: 10.1126/science.aac4690. PMID: 26430123. PMCID: PMC4725325.
Article61. Tritsch NX, Ding JB, Sabatini BL. 2012; Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature. 490:262–266. DOI: 10.1038/nature11466. PMID: 23034651. PMCID: PMC3944587.
Article62. Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TM, Allen JM, Mizumori SJ, Bonci A, Palmiter RD. 2011; Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 14:620–626. DOI: 10.1038/nn.2808. PMID: 21499253. PMCID: PMC3083461.
Article63. Bariselli S, Glangetas C, Tzanoulinou S, Bellone C. 2016; Ventral tegmental area subcircuits process rewarding and aversive experiences. J Neurochem. 139:1071–1080. DOI: 10.1111/jnc.13779. PMID: 27546491.
Article64. DeGroot SR, Zhao-Shea R, Chung L, Klenowski PM, Sun F, Molas S, Gardner PD, Li Y, Tapper AR. 2020; Midbrain dopamine controls anxiety-like behavior by engaging unique interpeduncular nucleus microcircuitry. Biol Psychiatry. 88:855–866. DOI: 10.1016/j.biopsych.2020.06.018. PMID: 32800629. PMCID: PMC8043246.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroanatomy of Sleep-Wake Regulation and its Application to Pharmacotherapy
- Behavioral and Histochemical Changes in MPTP-treated C57BL/6 Mice: A Model for Parkinson's Disease
- How Leptin Controls the Drive to Eat
- Beyond Substance Addiction: Broadening the Concept of Addiction to Include Behavioral Addiction
- Diffusion Measure Changes of Substantia Nigra Subregions and the Ventral Tegmental Area in Newly Diagnosed Parkinson’s Disease