Arch Hand Microsurg.
2022 Sep;27(3):211-216. 10.12790/ahm.22.0027.
Comparison of the hepatotoxicity of low-molecular-weight versus unfractionated heparin for anticoagulation therapy after digital replantation
- Affiliations
-
- 1Department of Plastic Surgery, Korea University Ansan Hospital, Ansan, Korea
- KMID: 2532736
- DOI: http://doi.org/10.12790/ahm.22.0027
Abstract
- Purpose
Unfractionated heparin (UFH) is more commonly used as an anticoagulant after digital replantation than low-molecular-weight heparin (LMWH). We compared the success and complication rates of these two anticoagulants, since only a few studies have made this comparison directly.
Methods
Forty-four patients who underwent digital replantation for complete or incomplete digital amputation in the past 7 years at a single institution were included. The patients were divided into LMWH and UFH groups according to the anticoagulant administered. The success rates for each group were obtained, and the postoperative serum aspartate aminotransferase (AST) and alanine transaminase (ALT) levels were analyzed to compare the complication rates.
Results
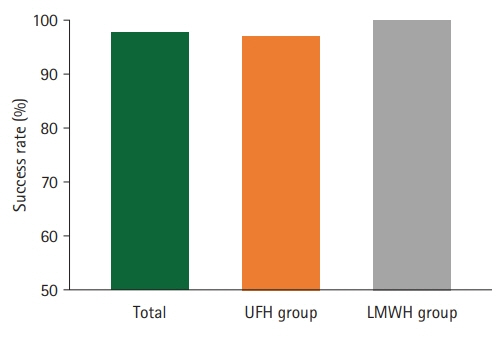
All patients, except one, had successful recovery of circulation after replantation, and the success rate did not show a statistically significant difference between the two groups. The statistical analysis showed that the proportion of patients with abnormal serum AST or ALT levels in the LMWH group was significantly lower than that in the UFH group.
Conclusion
Although there was no significant difference in the success rate between the two groups, the risk of hepatotoxicity was significantly lower in the LMWH group than in the UFH group. Considering the advantages of LMWH, its extensive use is highly recommended for anticoagulation therapy in patients after digital replantation.
Keyword
Figure
Reference
-
References
1. Kaciulyte J, Losco L, Maruccia M, et al. Postsurgical antithrombotic therapy in microsurgery: our protocol and literature review. Eur Rev Med Pharmacol Sci. 2019; 23:4448–57.2. Jokuszies A, Herold C, Niederbichler AD, Vogt PM. Anticoagulative strategies in reconstructive surgery: clinical significance and applicability. Ger Med Sci. 2012; 10:Doc01.3. Maeda M, Fukui A, Tamai S, Mizumoto S, Inada Y. Continuous local intra-arterial infusion of antithrombotic agents for replantation (comparison with intravenous infusion). Br J Plast Surg. 1991; 44:520–5.4. Pomerance J, Truppa K, Bilos ZJ, Vender MI, Ruder JR, Sagerman SD. Replantation and revascularization of the digits in a community microsurgical practice. J Reconstr Microsurg. 1997; 13:163–70.5. Rodríguez Vegas JM, Ruiz Alonso ME, Terán Saavedra PP. PGE-1 in replantation and free tissue transfer: early preliminary experience. Microsurgery. 2007; 27:395–7.6. Vretos KA, Tsavissis AG. Antithrombotic and antinflammatory drugs for protection of microvascular anastomosis. Acta Orthop Scand Suppl. 1995; 264:48–9.7. Lin PT, Wang SH, Chi CC. Low molecular weight heparin for prevention of microvascular occlusion in digital replantation. Cochrane Database Syst Rev. 2020; 4:CD009894.8. Baker EL, Loewenthal T, Salerno E, Baker WL. Probable enoxaparin-induced hepatotoxicity. Am J Health Syst Pharm. 2009; 66:638–41.9. Bosco M, Kish T. Hepatotoxicity with elevated bilirubin secondary to prophylactic doses of unfractionated heparin: a case report and review of heparin-induced hepatotoxicity. J Pharm Technol. 2019; 35:36–40.10. Carlson MK, Gleason PP, Sen S. Elevation of hepatic transaminases after enoxaparin use: case report and review of unfractionated and low-molecular-weight heparin-induced hepatotoxicity. Pharmacotherapy. 2001; 21:108–13.11. Haney JS, Carter CT, Downey J, Ilic R. A severe case of idiosyncratic hepatotoxicity with unfractionated heparin. Hosp Pharm. 2021; 56:777–85.12. AL-Mekhaizeem KA, Sherker AH. Heparin-induced hepatotoxicity. Can J Gastroenterol. 2001; 15:527–30.13. Zaera De La Fuente C, Arribas Anta J, López-San Román A, Cañete Ruiz Á, López Durán S. Enoxaparin-induced hepatotoxicity. Gastroenterol Hepatol. 2015; 38:438–9.14. Nozawa H, Emoto S, Sonoda H, et al. Liver injury among Japanese patients treated using prophylactic enoxaparin after colorectal surgery. Dig Dis Sci. 2021; 66:2805–15.15. Meneveau N. Safety evaluation of enoxaparin in currently approved indications. Expert Opin Drug Saf. 2009; 8:745–54.16. Gouin-Thibault I, Pautas E, Siguret V. Safety profile of different low-molecular weight heparins used at therapeutic dose. Drug Saf. 2005; 28:333–49.17. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med. 1998; 3:41–6.18. Solari F, Varacallo M. Low molecular weight heparin (LMWH) [updated 2022 Jul 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing;2020. [cited 2022 Jun 24]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525957/.19. Young E, Wells P, Holloway S, Weitz J, Hirsh J. Ex-vivo and in-vitro evidence that low molecular weight heparins exhibit less binding to plasma proteins than unfractionated heparin. Thromb Haemost. 1994; 71:300–4.20. Hirsh J. Low-molecular-weight heparin : a review of the results of recent studies of the treatment of venous thromboembolism and unstable angina. Circulation. 1998; 98:1575–82.21. Hahn KJ, Morales SJ, Lewis JH. Enoxaparin-induced liver injury: case report and review of the literature and FDA Adverse Event Reporting System (FAERS). Drug Saf Case Rep. 2015; 2:17.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful Treatment of Aortic Thrombosis after Umbilical Catheterization with Low-Molecular-Weight Heparin
- Portal vein thrombosis that successfully treated with low molecular weight heparin in acute pancreatitis
- Effect of standard heparin and low molecular weight heparin on fibrinolytic activity
- Enoxaparin-associated Retroperitoneal Hematoma in Two Chronic Dialysis Patients
- Successful Low Molecular Weight Heparin Treatment for the Global Alteration of Cortical Venous Drainage Developed after Intracranial Operation