J Korean Med Sci.
2022 Aug;37(32):e251. 10.3346/jkms.2022.37.e251.
Successful mRNA COVID-19 Vaccination and Colonoscopy After Oral Desensitization in a Patient With Polyethylene Glycol Allergy
- Affiliations
-
- 1Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea
- KMID: 2532325
- DOI: http://doi.org/10.3346/jkms.2022.37.e251
Abstract
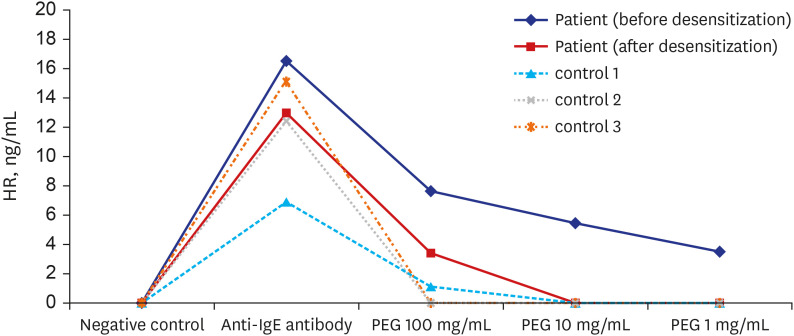
- Anaphylaxis to polyethylene glycol (PEG) is rare and mainly occurs with the use of laxatives containing PEG. Recently, an increasing number of PEG allergies have been reported, particularly those related to coronavirus disease 2019 (COVID-19) vaccines. mRNA COVID-19 vaccines, such as the BNT162b2 (Pfizer–BioNTech) and mRNA-1273 (Moderna) vaccines, contain PEG2000 as an excipient and are contraindicated when allergy to a vaccine component exist. We report a 55-year-old woman’s history as a case of successful mRNA COVID-19 vaccination and colonoscopy after oral desensitization to PEG in a patient with PEG allergy who required both COVID-19 vaccination and colon evaluation. Allergy to PEG was diagnosed based on clinical history, skin test results, and basophil histamine release testing. Oral desensitization effectively suppressed histamine release from basophils in response to PEG stimulation, suggesting that oral desensitization using PEG-based laxatives may be an effective treatment option for patients with allergy to the substance.
Figure
Reference
-
1. Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016; 46(7):907–922. PMID: 27196817.
Article2. Stone CA Jr, Liu Y, Relling MV, Krantz MS, Pratt AL, Abreo A, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019; 7(5):1533–1540.e8. PMID: 30557713.
Article3. Garvey LH, Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anaesth. 2021; 126(3):e106–e108. PMID: 33386124.
Article4. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020–January 18, 2021. JAMA. 2021; 325(11):1101–1102. PMID: 33576785.
Article5. Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, El-Gamal Y, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021; 14(2):100517. PMID: 33558825.
Article6. Kim MA, Lee YW, Kim SR, Kim JH, Min TK, Park HS, et al. COVID-19 vaccine-associated anaphylaxis and allergic reactions: consensus statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working Group. Allergy Asthma Immunol Res. 2021; 13(4):526–544. PMID: 34212542.
Article7. Klimek L, Jutel M, Akdis CA, Bousquet J, Akdis M, Torres MJ, et al. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines - an EAACI-ARIA Position Paper. Allergy. 2021; 76(6):1624–1628. PMID: 33378789.
Article8. Brockow K, Mathes S, Fischer J, Volc S, Darsow U, Eberlein B, et al. Experience with polyethylene glycol allergy-guided risk management for COVID-19 vaccine anaphylaxis. Allergy. 2022; 77(7):2200–2210. PMID: 34806775.
Article9. Kim KY, Kwon HJ, Cho SH, Nam M, Kim CW. Development and validation of a highly sensitive LC-MS/MS method for in vitro measurement of histamine concentration. J Pharm Biomed Anal. 2019; 172:33–41. PMID: 31022614.
Article10. Bruusgaard-Mouritsen MA, Jensen BM, Poulsen LK, Duus Johansen J, Garvey LH. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol. 2022; 149(1):168–175.e4. PMID: 34052265.
Article11. Zhou ZH, Stone CA Jr, Jakubovic B, Phillips EJ, Sussman G, Park J, et al. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2021; 9(4):1731–1733.e3. PMID: 33217616.
Article12. Korea Disease Control and Prevention Agency. Monitoring of adverse reactions after 3rd dose of COVID-19 vaccine. Public Health Wkly Rep. 2021; 14(52):3687–3693.13. Sellaturay P, Gurugama P, Harper V, Dymond T, Ewan P, Nasser S. The Polysorbate containing AstraZeneca COVID-19 vaccine is tolerated by polyethylene glycol (PEG) allergic patients. Clin Exp Allergy. 2022; 52(1):12–17. PMID: 34822190.
Article14. Bruusgaard-Mouritsen MA, Koo G, Heinrichsen AS, Melchiors BB, Krantz MS, Plager JH, et al. Janssen COVID-19 vaccine tolerated in 10 patients with confirmed polyethylene glycol allergy. J Allergy Clin Immunol Pract. 2022; 10(3):859–862. PMID: 34979336.
Article15. AlMuhizi F, Ton-Leclerc S, Fein M, Tsoukas C, Garvey LH, Lee D, et al. Successful desensitization to mRNA COVID-19 vaccine in a case series of patients with a history of anaphylaxis to the first vaccine dose. Front Allergy. 2022; 3:825164. PMID: 35386647.
Article16. Romantowski J, Kruszewski J, Solarski O, Bant A, Chciałowski A, Pietrzyk I, et al. Protocol of safe vaccination against COVID-19 in patients with high risk of allergic reactions. Clin Transl Allergy. 2022; 12(5):e12152. PMID: 35601631.
Article17. Paoletti G, Racca F, Piona A, Melone G, Merigo M, Puggioni F, et al. Successful SARS-CoV-2 vaccine allergy risk-management: the experience of a large Italian University Hospital. World Allergy Organ J. 2021; 14(5):100541. PMID: 33850601.
Article18. Banerji A, Wickner PG, Saff R, Stone CA Jr, Robinson LB, Long AA, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021; 9(4):1423–1437. PMID: 33388478.
Article19. Iemoli E, Ortolani VG, Preziosi D, Caron L, Giardina C, Carlevatti V, et al. Failure of desensitization with Pfizer-BioNTech COVID-19 vaccine in two asthmatic patients. Eur Ann Allergy Clin Immunol. Forthcoming 2021. DOI: 10.23822/EurAnnACI.1764-1489.236.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal and Safe Bowel Preparation for Colonoscopy
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning
- A Randomized Prospective Trial Comparing a New Polyethylene Glycol Based Lavage Solution with the Standard Polyethylene Glycol Solution in the Preparation of Patients Undergoing Colonoscopy (Clinical trial of new PEG solution in bowel preparation)
- Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination
- A Case of Ischemic Colitis by Oral Sulfate Free-Polyethylene Glycol