J Korean Med Sci.
2022 Jul;37(29):e228. 10.3346/jkms.2022.37.e228.
Clinical Characteristics of COVID-19: Use of Steroids in Mostly Unvaccinated COVID-19 Patients Before the Omicron Variant
- Affiliations
-
- 1Department of Internal Medicine, Jeonbuk National University Medical School and Hospital, Jeonju, Korea
- 2Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 3Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, Korea
- 4Department of Internal Medicine, Korea Cancer Center Hospital, Seoul, Korea
- KMID: 2532085
- DOI: http://doi.org/10.3346/jkms.2022.37.e228
Abstract
- Background
Glucocorticoids are one of the current standard agents for moderate to severe coronavirus disease 2019 (COVID-19) treatment based on the RECOVERY trial. Data on the real clinical application of steroids for COVID-19 are scarce and will help guide the optimal use of steroids. We described the current prescription pattern of steroids for COVID-19 and investigated the factors related to specific practices.
Methods
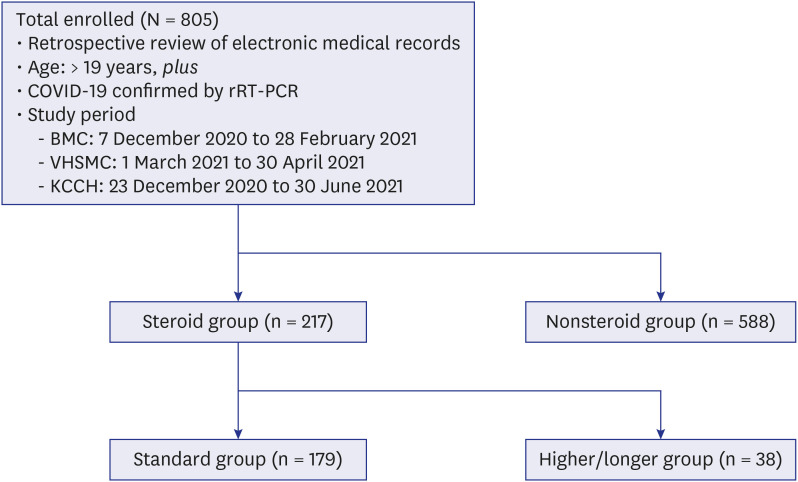
All adults aged ≥ 19 years who were diagnosed with COVID-19 by real-time reverse transcription-polymerase chain reaction and admitted to one of 3 study hospitals from 8 December 2020 to 30 June 2021 were enrolled. Demographic and clinical data, including medications and oxygen therapy, were retrospectively collected from electronic medical records. The severity of comorbidities and COVID-19 were measured. The subjects were divided into steroid and nonsteroid groups, and the steroid group was then subdivided into standard and higher/longer groups.
Results
Among a total of 805 patients, 217 (27.0%) were treated with steroids. The steroid group showed a higher rate of oxygen therapy (81.1% vs. 2.7%), more concomitant use of remdesivir (77.4% vs. 1.4%) or antibiotics (79.3% vs. 4.3%), and a higher proportion of high risk according to National Early Warning Score-2 score (30.0% vs. 0.9%) or severe risk according to National Institute of Allergy and Infectious Disease Ordinal Scale score (81.1% vs. 2.7%) than the nonsteroid group. The mortality of the steroid group was 4.6%. In the steroid group, 82.5% received a standard or lower dose of steroids within ten days, and 17.5% (38/217) received a higher or longer dose of steroids. Multivariate analysis showed that initial lymphopenia (adjusted odds ratio [aOR], 0.94; 95% confidence interval [CI], 0.89–0.99) and high level of lactate dehydrogenase (LDH) (aOR, 1.00; 95% CI, 1.00–1.01) were independent risk factors for higher doses or longer steroid use.
Conclusion
The dose and duration of steroids were in line with current guidelines in 82.5% of COVID-19 patients, but the outliers may need tailored therapy according to surrogate markers, such as initial lymphopenia or high level of LDH.
Keyword
Figure
Cited by 1 articles
-
Is Longer Duration or Higher Dose of Steroid Use Effective in Critical COVID-19 Patients?: An Area of Uncertainty
Won Suk Choi
J Korean Med Sci. 2022;37(29):e242. doi: 10.3346/jkms.2022.37.e242.
Reference
-
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382(8):727–733. PMID: 31978945.
Article2. World Health Organization. Coronavirus Disease (COVID-2019) Situation Reports. Geneva, Switzerland: World Health Organization;2020.3. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30(3):269–271. PMID: 32020029.
Article4. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - Final report. N Engl J Med. 2020; 383(19):1813–1826. PMID: 32445440.
Article5. NIH’s therapeutic management of hospitalized adults with COVID-19. Updated December 16, 2021. Accessed February 10, 2022. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management .6. IDSA’s guidelines on the treatment and management of patients with COVID-19. Updated February 8, 2022. Accessed February 10, 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management .7. Agarwal A, Rochwerg B, Lamontagne F, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020; 370:m3379. PMID: 32887691.8. Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021; 9(12):1407–1418. PMID: 34480861.9. RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021; 384(8):693–704. PMID: 32678530.
Article10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40(5):373–383. PMID: 3558716.
Article11. Royal College of Physicians’ National Early Warning Scorre (NEWS) 2. Updated December 19, 2017. Accessed February 10, 2022. https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 .12. Goletti D, Cantini F. Baricitinib therapy in Covid-19 pneumonia - an unmet need fulfilled. N Engl J Med. 2021; 384(9):867–869. PMID: 33657299.
Article13. Vita S, Centanni D, Lanini S, Piselli P, Rosati S, Giancola ML, et al. Benefits of steroid therapy in COVID-19 patients with different PaO2/FiO2 ratio at admission. J Clin Med. 2021; 10(15):3236. PMID: 34362021.
Article14. Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021; 27(1):83–88. PMID: 32745596.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mathematical modeling of the impact of Omicron variant on the COVID-19 situation in South Korea
- The Impact of Omicron Wave on Pediatric Febrile Seizure
- Effectiveness of the Bivalent mRNA COVID-19 Vaccine for Preventing Critical Infection From the SARS-CoV-2 Omicron Variant in the Republic of Korea
- Osteonecrosis following Steroid Therapy in COVID-19 Patients: An Outlook on the Emerging Problem
- Descriptive analysis of the incidence rate of post-acute COVID-19 syndrome in the Republic of Korea Army