J Neurocrit Care.
2022 Jun;15(1):21-31. 10.18700/jnc.210031.
Use of temperature changes and pro-inflammatory biomarkers to diagnose bacterial infections in patients with severe cerebral trauma
- Affiliations
-
- 1Faculty of Medicine and Health Sciences, Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden
- 2Faculty of Medicine and Health Sciences, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden
- 3Department of Anaesthesiology and Intensive Care, University Hospital, Linköping, Sweden
- KMID: 2531996
- DOI: http://doi.org/10.18700/jnc.210031
Abstract
- Background
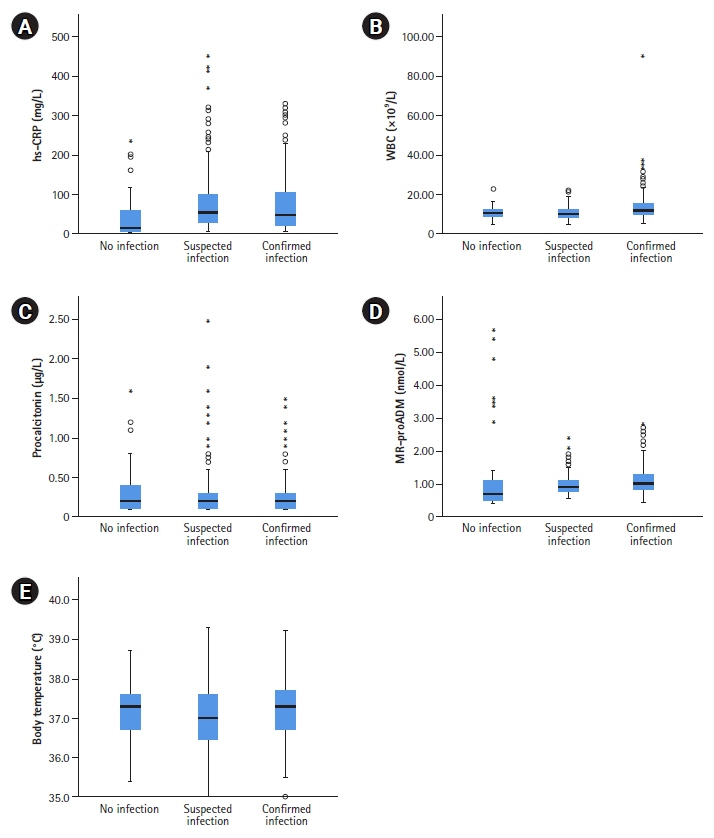
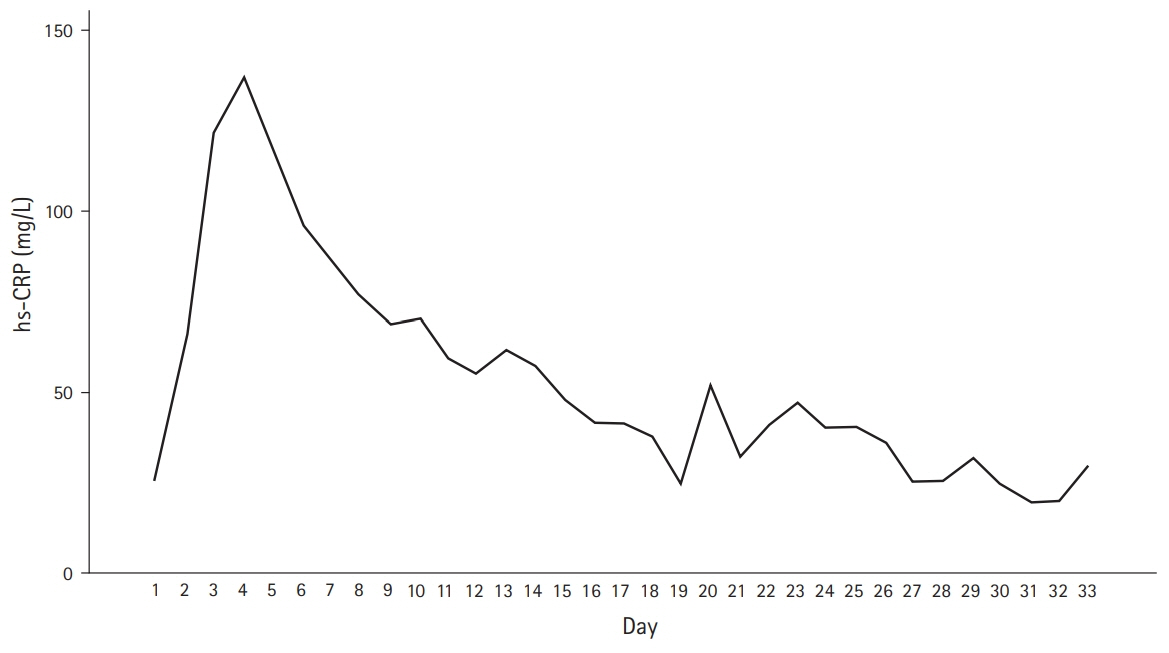
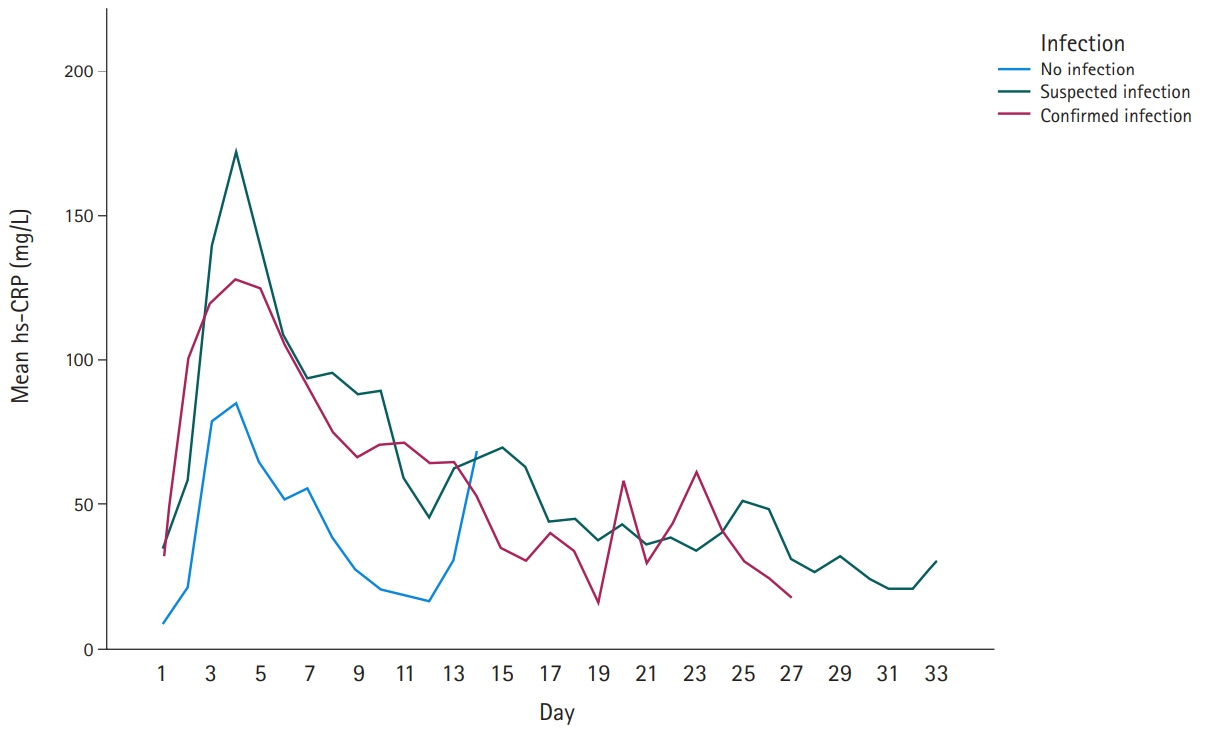
In patients undergoing neurosurgeries, inflammation and infection are strongly related; however, inflammation can be present without infection. Midregional proadrenomedullin (MR-proADM) is a relatively new sepsis biomarker that is rarely used clinically. Recently, the concept of DiffTemp was introduced, that is, a >1°C rise from individual normal temperature accompanied by malaise, as a more accurate definition of temperature assessed as fever. The aim of the present study was to examine the importance of C-reactive protein (CRP), white blood cells, procalcitonin, and MR-proADM levels and DiffTemp.
Methods
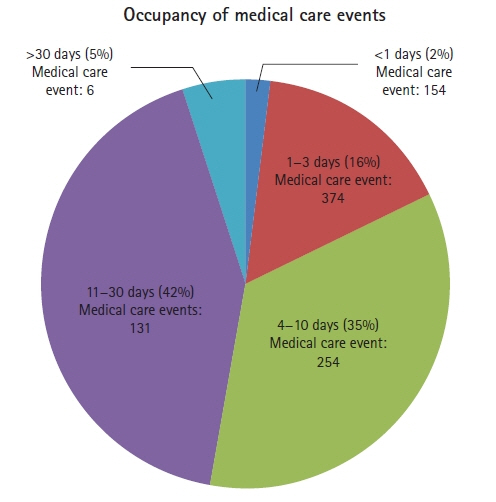
This prospective, comparative study had a quantitative approach. Forty-two patients, aged >18 years and presenting with severe cerebral trauma were included from a neurosurgery intensive care unit. The outcome variable was infection; group 0, no infection (n=11); group 1, suspected infection (n=15); and, group 2, confirmed infection (n=16). Group assignments were performed using biomarkers, medical records, bacterial cultures, and International Classification of Diseases-10, and by the clinical assessment of criteria for nosocomial infections by a neurosurgeon.
Results
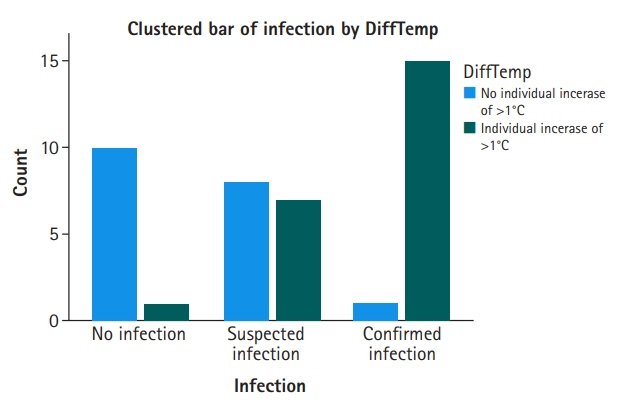
On comparing groups 1 and 2, MR-proADM and DiffTemp were associated with a higher risk of confirmed infection (odds ratio, 5.41 and 17.14, respectively). Additionally, DiffTemp had a 90.9% specificity in patients with no infection and a 93.8% sensitivity in patients with confirmed infections. CRP and procalcitonin levels were not associated with an increased risk of confirmed infection.
Conclusion
Increased levels of MR-proADM were associated with a higher risk of confirmed infection. DiffTemp was associated with a higher risk of having a confirmed infection.
Figure
Reference
-
1. Godbolt AK, Stenberg M, Jakobsson J, Sorjonen K, Krakau K, Stålnacke BM, et al. Subacute complications during recovery from severe traumatic brain injury: frequency and associations with outcome. BMJ Open. 2015; 5:e007208.
Article2. Godbolt AK, Stenberg M, Lindgren M, Ulfarsson T, Lannsjö M, Stålnacke BM, et al. Associations between care pathways and outcome 1 year after severe traumatic brain injury. J Head Trauma Rehabil. 2015; 30:E41–51.
Article3. Goyal K, Garg N, Bithal P. Central fever: a challenging clinical entity in neurocritical care. J Neurocrit Care. 2020; 13:19–31.
Article4. Sajid MS, Shakir AJ, Khatri K, Baig MK. The role of perioperative warming in surgery: a systematic review. Sao Paulo Med J. 2009; 127:231–7.
Article5. Drewry AM, Fuller BM, Bailey TC, Hotchkiss RS. Body temperature patterns as a predictor of hospital-acquired sepsis in afebrile adult intensive care unit patients: a case-control study. Crit Care. 2013; 17:R200.
Article6. Grodzinsky E, Levander MS. Understanding fever and body temperature: a cross-disciplinary approach to clinical practice. New York, NY: Palgrave McMillan;2020.7. Mackowiak PA. Clinical thermometric measurements. In : Mackowiak PA, editor. Fever: basic mechanisms and management. Philadelphia, PA: Lippincott Raven;1997. p. 27–33.8. Chamberlain JM, Terndrup TE, Alexander DT, Silverstone FA, Wolf-Klein G, O’Donnell R, et al. Determination of normal ear temperature with an infrared emission detection thermometer. Ann Emerg Med. 1995; 25:15–20.
Article9. Sund-Levander M, Grodzinsky E, Loyd D, Wahren LK. Errors in body temperature assessment related to individual variation, measuring technique and equipment. Int J Nurs Pract. 2004; 10:216–23.
Article10. Levander MS, Grodzinsky E. Variation in normal ear temperature. Am J Med Sci. 2017; 354:370–8.
Article11. McCarthy PW, Heusch AI. The vagaries of ear temperature assessment. J Med Eng Technol. 2006; 30:242–51.
Article12. Ring EF, McEvoy H, Jung A, Zuber J, Machin G. New standards for devices used for the measurement of human body temperature. J Med Eng Technol. 2010; 34:249–53.
Article13. Geijer H, Udumyan R, Lohse G, Nilsagård Y. Temperature measurements with a temporal scanner: systematic review and meta-analysis. BMJ Open. 2016; 6:e009509.
Article14. Kiekkas P, Stefanopoulos N, Bakalis N, Kefaliakos A, Karanikolas M. Agreement of infrared temporal artery thermometry with other thermometry methods in adults: systematic review. J Clin Nurs. 2016; 25:894–905.
Article15. Mackowiak PA, Worden G. Carl Reinhold August Wunderlich and the evolution of clinical thermometry. Clin Infect Dis. 1994; 18:458–67.
Article16. Mackowiak PA. Fever’s upper limit. In : Mackowiak PA, editor. Fever: basic mechanisms and management. Philadelphia, PA: Lippincott Raven;1997. p. 147–63.17. Sund-Levander M, Grodzinsky E. Time for a change to assess and evaluate body temperature in clinical practice. Int J Nurs Pract. 2009; 15:241–9.
Article18. Levander MS, Tingström P. Fever or not fever–that’s the question: a cohort study of simultaneously measured rectal and ear temperatures in febrile patients with suspected infection. Clin Nurs Stud. 2018; 6:47–54.19. Sweden’s municipalities and regions. Nosocomial infections (Sveriges Kommuner och Regioner (SKR), Vårdrelaterade infektioner) [Internet]. Stockholm: SKR;2021. [cited 2021 Dec 8]. Available from: https://webbutik.skr.se/bilder/artiklar/pdf/7585-475-5.pdf.20. National Board of Health and Welfare. ICD-10-SE [Internet]. Stockholm: Socialstyrelsen;2021. [cited 2021 Dec 8]. Available from: https://www.socialstyrelsen.se/utveckla-verksamhet/e-halsa/klassificering-och-koder/icd-10/.21. Dippel DW, van Breda EJ, van Gemert HM, van der Worp HB, Meijer RJ, Kappelle LJ, et al. Effect of paracetamol (acetaminophen) on body temperature in acute ischemic stroke: a double-blind, randomized phase II clinical trial. Stroke. 2001; 32:1607–12.
Article22. Forrest JA, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet. 1982; 7:93–107.
Article23. de la Rubia de la Rubia JA, Aguirre-Jaime A, Fernández Vilar AM, Pérez Higuera C. Tympanic thermometer. The left or the right ear? Rev Enferm. 2002; 25:50–4.24. Nehring SM, Goyal A, Patel BC. C reactive protein [Internet]. Treasure Island, FL: StatPearls Publishing;2022. [cited 2021 Dec 8]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441843/.25. Liu HH, Zhang MW, Guo JB, Li J, Su L. Procalcitonin and C-reactive protein in early diagnosis of sepsis caused by either Gram-negative or Gram-positive bacteria. Ir J Med Sci. 2017; 186:207–12.
Article26. Póvoa P. C-reactive protein: a valuable marker of sepsis. Intensive Care Med. 2002; 28:235–43.
Article27. Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983; 34:141–212.
Article28. Riley LK, Rupert J. Evaluation of patients with leukocytosis. Am Fam Physician. 2015; 92:1004–11.29. Jensen JU, Heslet L, Jensen TH, Espersen K, Steffensen P, Tvede M. Procalcitonin increase in early identification of critically ill patients at high risk of mortality. Crit Care Med. 2006; 34:2596–602.
Article30. Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med. 2013; 28:285–91.
Article31. Sager R, Kutz A, Mueller B, Schuetz P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017; 15:15.
Article32. Sudhir U, Venkatachalaiah RK, Kumar TA, Rao MY, Kempegowda P. Significance of serum procalcitonin in sepsis. Indian J Crit Care Med. 2011; 15:1–5.
Article33. Jekarl DW, Lee S, Kim M, Kim Y, Woo SH, Lee WJ. Procalcitonin as a prognostic marker for sepsis based on SEPSIS-3. J Clin Lab Anal. 2019; 33:e22996.
Article34. Taylor R, Jones A, Kelly S, Simpson M, Mabey J. A review of the value of procalcitonin as a marker of infection. Cureus. 2017; 9:e1148.
Article35. Pelinka LE, Petto H, Kroepfl A, Schmidhammer R, Redl H. Serum procalcitonin and S100B are associated with mortality after traumatic brain injury. Eur J Trauma. 2003; 29:316–22.
Article36. Oconnor E, Venkatesh B, Mashongonyika C, Lipman J, Hall J, Thomas P. Serum procalcitonin and C-reactive protein as markers of sepsis and outcome in patients with neurotrauma and subarachnoid haemorrhage. Anaesth Intensive Care. 2004; 32:465–70.
Article37. Sinaga B, Mahadewa TG, Maliawan AS. High blood levels procalcitonin as systemic imflamatory response syndrome predictor in severe and moderate head injury. Bali Med J. 2014; 3:25–30.
Article38. Elsasser TH, Kahl S. Adrenomedullin has multiple roles in disease stress: development and remission of the inflammatory response. Microsc Res Tech. 2002; 57:120–9.
Article39. Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, et al. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001; 276:12292–300.
Article40. Önal U, Valenzuela-Sánchez F, Vandana KE, Rello J. Mid-regional pro-adrenomedullin (MR-proADM) as a biomarker for sepsis and septic shock: narrative review. Healthcare (Basel). 2018; 6:110.
Article41. Wolkewitz M, Schumacher M, Rücker G, Harbarth S, Beyersmann J. Estimands to quantify prolonged hospital stay associated with nosocomial infections. BMC Med Res Methodol. 2019; 19:111.
Article42. Saviteer SM, Samsa GP, Rutala WA. Nosocomial infections in the elderly. Increased risk per hospital day. Am J Med. 1988; 84:661–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of the Neutrophil-to-Lymphocyte Ratio as a Predictor of Prognosis in Cellulitis
- Exertional heat stroke with reversible severe cerebral edema
- Changes of Biomarkers before and after Antibiotic Treatment in Spinal Infection
- Fever in Trauma Patients without Brain Injury
- Genetics and Biomarkers of Moyamoya Disease: Significance of RNF213 as a Susceptibility Gene