Diabetes Metab J.
2022 Jul;46(4):543-551. 10.4093/dmj.2022.0209.
Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- KMID: 2531958
- DOI: http://doi.org/10.4093/dmj.2022.0209
Abstract
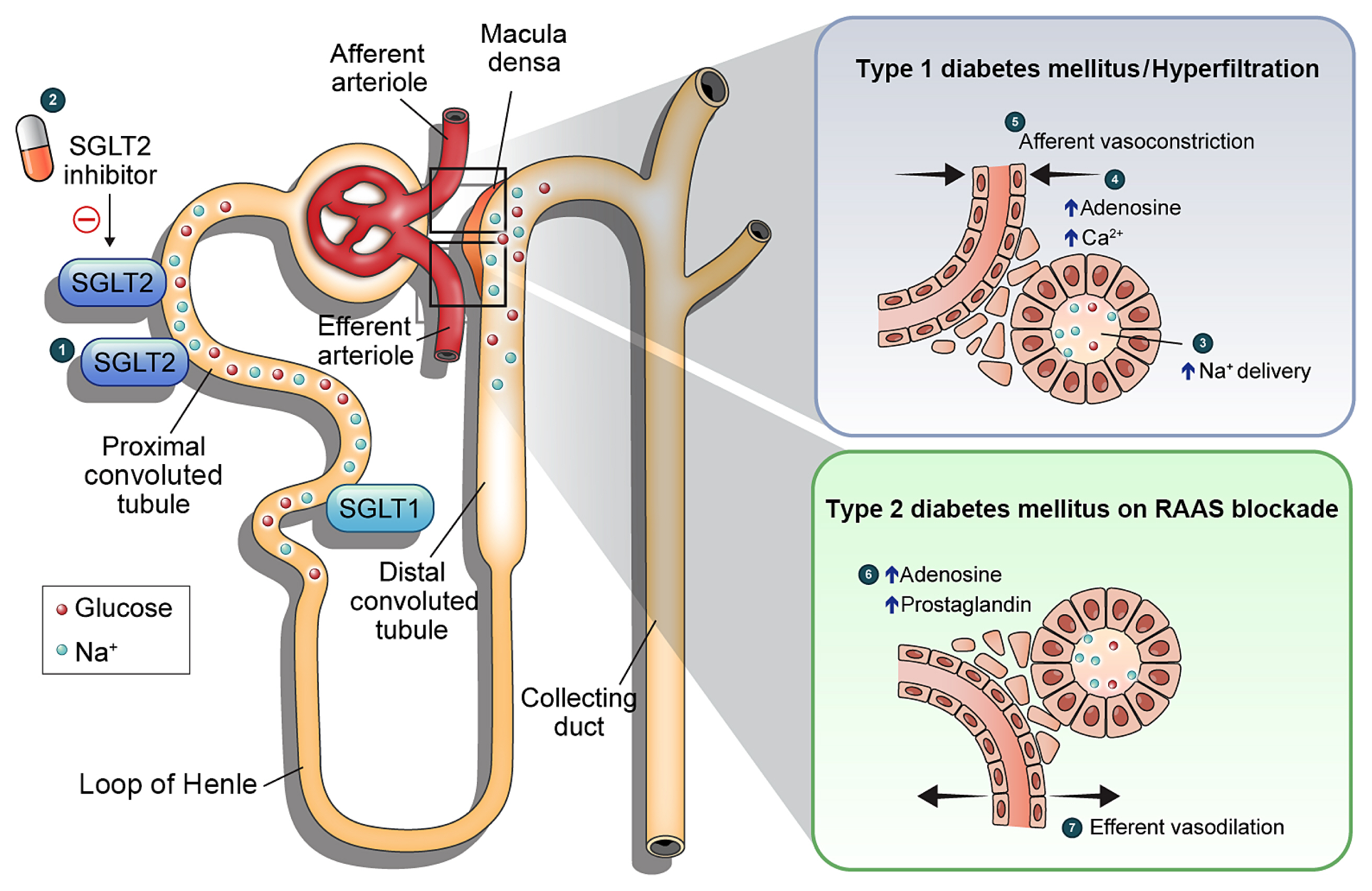
- Diabetic kidney disease (DKD) is a prevalent renal complication of diabetes mellitus that ultimately develops into end-stage kidney disease (ESKD) when not managed appropriately. Substantial risk of ESKD remains even with intensive management of hyperglycemia and risk factors of DKD and timely use of renin-angiotensin-aldosterone inhibitors. Sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce hyperglycemia primarily by inhibiting glucose and sodium reabsorption in the renal proximal tubule. Currently, their effects expand to prevent or delay cardiovascular and renal adverse events, even in those without diabetes. In dedicated renal outcome trials, SGLT2 inhibitors significantly reduced the risk of composite renal adverse events, including the development of ESKD or renal replacement therapy, which led to the positioning of SGLT2 inhibitors as the mainstay of chronic kidney disease management. Multiple mechanisms of action of SGLT2 inhibitors, including hemodynamic, metabolic, and anti-inflammatory effects, have been proposed. Restoration of tubuloglomerular feedback is a plausible explanation for the alteration in renal hemodynamics induced by SGLT2 inhibition and for the associated renal benefit. This review discusses the clinical rationale and mechanism related to the protection SGLT2 inhibitors exert on the kidney, focusing on renal hemodynamic effects.
Keyword
Figure
Cited by 3 articles
-
SGLT2 Inhibitors and Diabetes: Where Does It Come from and Where Does It Go?
Ji Yoon Kim, Sin Gon Kim
J Korean Diabetes. 2024;25(1):9-15. doi: 10.4093/jkd.2024.25.1.9.Cardiovascular Disease & Diabetes Statistics in Korea: Nationwide Data 2010 to 2019
Jin Hwa Kim, Junyeop Lee, Kyungdo Han, Jae-Taek Kim, Hyuk-Sang Kwon
Diabetes Metab J. 2024;48(6):1084-1092. doi: 10.4093/dmj.2024.0275.Prevalence, Incidence, and Metabolic Characteristics of Young Adults with Type 2 Diabetes Mellitus in South Korea (2010–2020)
Ji Yoon Kim, Jiyoon Lee, Joon Ho Moon, Se Eun Park, Seung-Hyun Ko, Sung Hee Choi, Nam Hoon Kim
Diabetes Metab J. 2025;49(2):172-182. doi: 10.4093/dmj.2024.0826.
Reference
-
1. Atkins RC. The epidemiology of chronic kidney disease. Kidney Int Suppl. 2005; 94:S14–8.
Article2. The Korean Society of Nephrology: Trends in epidemiologic characteristics of end-stage renal disease from 2020. KORDS (Korean Renal Data System);Available from: https://ksn.or.kr/(cited 2022 Jul 15).3. Ahn JH, Yu JH, Ko SH, Kwon HS, Kim DJ, Kim JH, et al. Prevalence and determinants of diabetic nephropathy in Korea: Korea National Health and Nutrition Examination Survey. Diabetes Metab J. 2014; 38:109–19.
Article4. Kim KJ, Kwon TY, Yu S, Seo JA, Kim NH, Choi KM, et al. Ten-year mortality trends for adults with and without diabetes mellitus in South Korea, 2003 to 2013. Diabetes Metab J. 2018; 42:394–401.
Article5. Lee MJ, Ha KH, Kim DJ, Park I. Trends in the incidence, prevalence, and mortality of end-stage kidney disease in South Korea. Diabetes Metab J. 2020; 44:933–7.
Article6. Park JH, Ha KH, Kim BY, Lee JH, Kim DJ. Trends in cardiovascular complications and mortality among patients with diabetes in South Korea. Diabetes Metab J. 2021; 45:120–4.
Article7. Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am. 2013; 97:1–18.
Article8. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001; 345:861–9.
Article9. Heart outcomes prevention evaluation study investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000; 355:253–9.10. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001; 345:851–60.
Article11. Sawaf H, Thomas G, Taliercio JJ, Nakhoul G, Vachharajani TJ, Mehdi A. Therapeutic advances in diabetic nephropathy. J Clin Med. 2022; 11:378.
Article12. Yamazaki T, Mimura I, Tanaka T, Nangaku M. Treatment of diabetic kidney disease: current and future. Diabetes Metab J. 2021; 45:11–26.
Article13. Coady MJ, Wallendorff B, Lapointe JY. Characterization of the transport activity of SGLT2/MAP17, the renal low-affinity Na+-glucose cotransporter. Am J Physiol Renal Physiol. 2017; 313:F467–74.14. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007; 261:32–43.
Article15. Tabatabai NM, Sharma M, Blumenthal SS, Petering DH. Enhanced expressions of sodium-glucose cotransporters in the kidneys of diabetic Zucker rats. Diabetes Res Clin Pract. 2009; 83:e27–30.
Article16. Norton L, Shannon CE, Fourcaudot M, Hu C, Wang N, Ren W, et al. Sodium-glucose co-transporter (SGLT) and glucose transporter (GLUT) expression in the kidney of type 2 diabetic subjects. Diabetes Obes Metab. 2017; 19:1322–6.
Article17. Abdul-Ghani MA, DeFronzo RA. Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract. 2008; 14:782–90.
Article18. Tsujihara K, Hongu M, Saito K, Inamasu M, Arakawa K, Oku A, et al. Na(+)-glucose cotransporter inhibitors as antidiabetics: I. synthesis and pharmacological properties of 4′-dehydroxyphlorizin derivatives based on a new concept. Chem Pharm Bull (Tokyo). 1996; 44:1174–80.
Article19. Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG outcome study. Diabetes Care. 2016; 39:717–25.
Article20. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020; 17:761–72.
Article21. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–9.
Article22. Neuen BL, Young T, Heerspink HJ, Neal B, Perkovic V, Billot L, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019; 7:845–54.
Article23. Kim HJ, Kim SS, Song SH. Glomerular filtration rate as a kidney outcome of diabetic kidney disease: a focus on new antidiabetic drugs. Korean J Intern Med. 2022; 37:502–19.
Article24. Sen T, Heerspink HJ. A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors. Cell Metab. 2021; 33:732–9.
Article25. DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021; 17:319–34.
Article26. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–28.
Article27. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016; 375:323–34.
Article28. Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol. 2018; 6:691–704.
Article29. Mosenzon O, Wiviott SD, Cahn A, Rozenberg A, Yanuv I, Goodrich EL, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019; 7:606–17.
Article30. Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–46.
Article31. Wheeler DC, Stefansson BV, Jongs N, Chertow GM, Greene T, Hou FF, et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021; 9:22–31.
Article32. Wanner C, Heerspink HJL, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG outcome trial. J Am Soc Nephrol. 2018; 29:2755–69.
Article33. Levin A, Perkovic V, Wheeler DC, Hantel S, George JT, von Eynatten M, et al. Empagliflozin and cardiovascular and kidney outcomes across KDIGO risk categories: post hoc analysis of a randomized, double-blind, placebo-controlled, multinational trial. Clin J Am Soc Nephrol. 2020; 15:1433–44.34. Heerspink HJ, Cherney DZ. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol. 2021; 16:1278–80.
Article35. Oh TJ, Moon JY, Hur KY, Ko SH, Kim HJ, Kim T, et al. Sodium-glucose cotransporter-2 inhibitor for renal function preservation in patients with type 2 diabetes mellitus: a Korean Diabetes Association and Korean Society of Nephrology consensus statement. Diabetes Metab J. 2020; 44:489–97.
Article36. Cherney DZI, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley RE, Frederich R, et al. Initial eGFR changes with ertugliflozin and associations with clinical parameters: analyses from the VERTIS CV trial. Am J Nephrol. 2022; 1–10.
Article37. Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, et al. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021; 99:999–1009.
Article38. Menne J, Dumann E, Haller H, Schmidt BM. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med. 2019; 16:e1002983.
Article39. McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008.40. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383:1413–24.41. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385:1451–61.42. van der Aart-van der Beek AB, de Boer RA, Heerspink HJ. Kidney and heart failure outcomes associated with SGLT2 inhibitor use. Nat Rev Nephrol. 2022; 18:294–306.
Article43. Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol. 2017; 28:1023–39.
Article44. Bak M, Thomsen K, Christiansen T, Flyvbjerg A. Renal enlargement precedes renal hyperfiltration in early experimental diabetes in rats. J Am Soc Nephrol. 2000; 11:1287–92.
Article45. Jung CY, Yoo TH. Pathophysiologic mechanisms and potential biomarkers in diabetic kidney disease. Diabetes Metab J. 2022; 46:181–97.
Article46. Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010; 53:2093–104.
Article47. Vora JP, Dolben J, Dean JD, Thomas D, Williams JD, Owens DR, et al. Renal hemodynamics in newly presenting non-insulin dependent diabetes mellitus. Kidney Int. 1992; 41:829–35.
Article48. Keller CK, Bergis KH, Fliser D, Ritz E. Renal findings in patients with short-term type 2 diabetes. J Am Soc Nephrol. 1996; 7:2627–35.
Article49. Thomson SC, Blantz RC. Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J Am Soc Nephrol. 2008; 19:2272–5.50. Vallon V. Tubuloglomerular feedback and the control of glomerular filtration rate. News Physiol Sci. 2003; 18:169–74.
Article51. Bank N, Aynedjian HS. Progressive increases in luminal glucose stimulate proximal sodium absorption in normal and diabetic rats. J Clin Invest. 1990; 86:309–16.
Article52. Ferrannini E, Veltkamp SA, Smulders RA, Kadokura T. Renal glucose handling: impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2013; 36:1260–5.53. Vervoort G, Veldman B, Berden JH, Smits P, Wetzels JF. Glomerular hyperfiltration in type 1 diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur J Clin Invest. 2005; 35:330–6.
Article54. Pruijm M, Wuerzner G, Maillard M, Bovet P, Renaud C, Bochud M, et al. Glomerular hyperfiltration and increased proximal sodium reabsorption in subjects with type 2 diabetes or impaired fasting glucose in a population of the African region. Nephrol Dial Transplant. 2010; 25:2225–31.
Article55. Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999; 10:2569–76.56. Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol. 2012; 74:351–75.
Article57. Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017; 24:73–9.
Article58. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019; 139:2022–31.
Article59. Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012; 302:R75–83.
Article60. Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014; 129:587–97.
Article61. Kidokoro K, Cherney DZ, Bozovic A, Nagasu H, Satoh M, Kanda E, et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019; 140:303–15.
Article62. Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int. 1996; 49:1774–7.
Article63. Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981; 241:F85–93.
Article64. Alonso-Galicia M, Dwyer TM, Herrera GA, Hall JE. Increased hyaluronic acid in the inner renal medulla of obese dogs. Hypertension. 1995; 25(4 Pt 2):888–92.
Article65. Henegar JR, Bigler SA, Henegar LK, Tyagi SC, Hall JE. Functional and structural changes in the kidney in the early stages of obesity. J Am Soc Nephrol. 2001; 12:1211–7.
Article66. van Bommel EJ, Muskiet MH, van Baar MJ, Tonneijck L, Smits MM, Emanuel AL, et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020; 97:202–12.
Article67. Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006; 86:901–40.
Article68. Ott C, Kannenkeril D, Jung S, Schnieder R. Combination therapy of empagliflozin and linagliptin vs. metformin and insulin glargine on intra- and renal hemodynamics in type 2 diabetes. ASN Kidney Week. 2019. Nov. 5. –10. Washington, DC: Available from: https://www.asn-online.org/education/kidneyweek/2019/program-abstract.aspx?controlId=3233843 .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sodium-Glucose Cotransporter 2 Inhibitors: Mechanisms of Action and Various Effects
- Sodium-Glucose Cotransporter 2 Inhibitors for Chronic Kidney Disease: A Comprehensive Review
- The Potential Cardioprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors
- Sodium-Glucose Cotransporter 2 Inhibitors for People with Type 1 Diabetes
- Recent Advances in Sodium Glucose Cotransporter-2 Inhibitors