Obstet Gynecol Sci.
2022 Jul;65(4):335-345. 10.5468/ogs.22025.
Human papillomavirus genotype distribution in low-grade squamous intraepithelial lesion cytology, and its immediate risk for high-grade cervical lesion or cancer: a single-center, cross-sectional study
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 2Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- KMID: 2531842
- DOI: http://doi.org/10.5468/ogs.22025
Abstract
Objective
To investigate the distribution of human papillomavirus (HPV) genotypes in low-grade squamous intraepithelial lesion (LSIL) cytology and the immediate risk of cervical intraepithelial neoplasia grade 2 or higher (CIN2+) lesions.
Methods
This prospective cross-sectional study enrolled women aged ≥21 years that were diagnosed with LSIL cytology at Siriraj Hospital (Bangkok, Thailand) during 2017-2019. Anyplex II HPV testing was performed to detect 14 high-risk HPV cases prior to colposcopy-directed biopsy.
Results
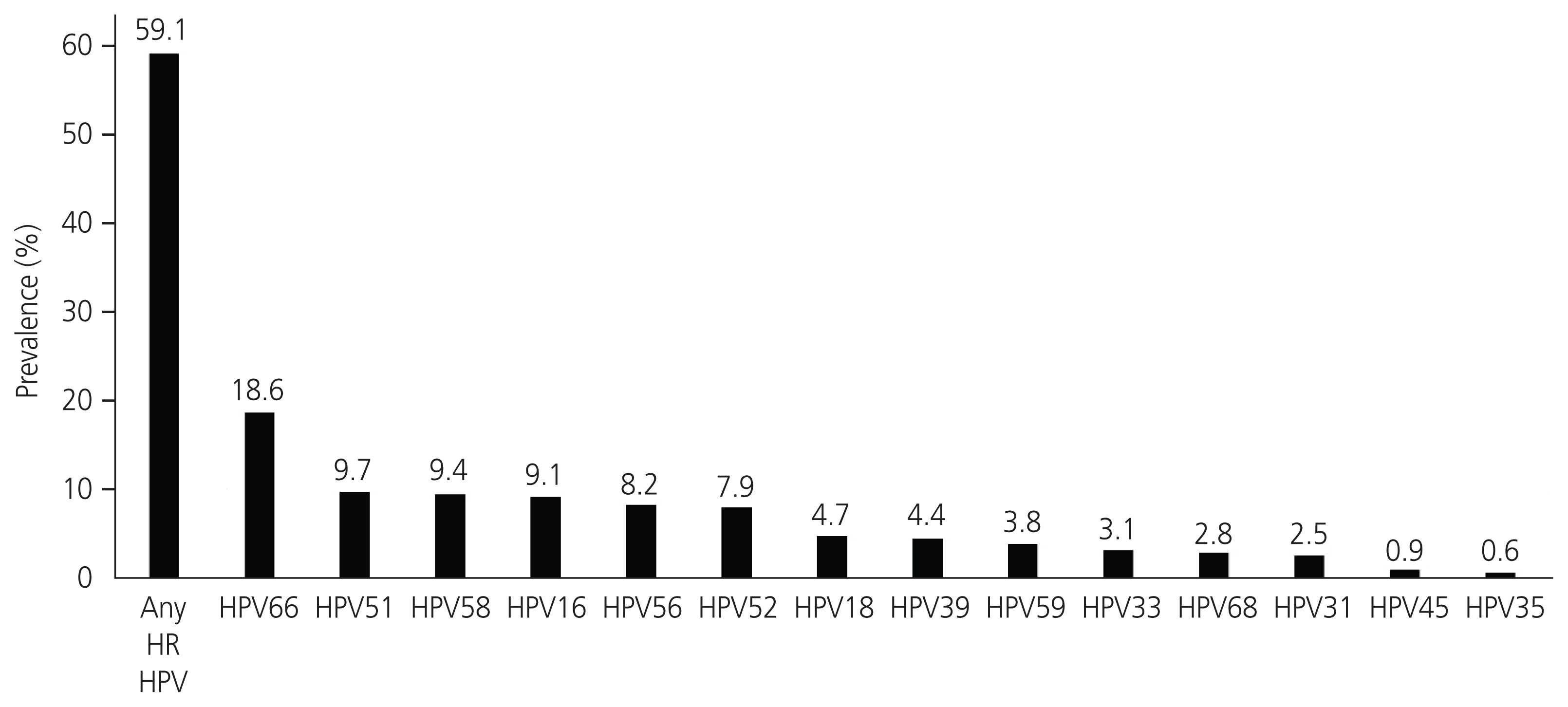
In total, 318 patients were included in the final analysis. Of those, 24 (7.5%), 241 (75.8%), 53 (16.7%) were aged 21- 25 years, 25-50 years, and ≥50 years, respectively. Eighty-two patients (25.8%) had abnormal screening results within the previous 5 years. High-risk HPV infection was found in 188 patients (59.1%) with 127 (39.9%) having single and 61 (19.2%) having multiple infections. The five most common HPV genotypes were HPV 66 (18.6%), HPV51 (9.7%), HPV58 (9.4%), HPV16 (9.1%), and HPV56 (8.2%). The immediate risk of CIN2+ was 6% in LSIL, regardless of the HPV status, 8% in high-risk HPV-positive LSIL, and 3.1% in high-risk HPV-negative LSIL. When using 6% as the threshold risk for colposcopy, performing reflex HPV testing in LSIL cytology can decrease the number of colposcopies by 40.9%, with an area under the receiver operating characteristic curve of 0.6 (95% confidence interval, 0.5-0.7).
Conclusion
The study findings support the idea that geographic variations affect the HPV genotype. Reflex HPV testing may decrease the number of colposcopies in cytology-based screening regions with a high prevalence of low-carcinogenic HPV.
Figure
Cited by 1 articles
-
Significance and limitations of routine p16/Ki-67 immunohistochemistry as a diagnostic tool for high-grade squamous intraepithelial lesions of the uterine cervix
Kazutaka Ozono, Fumi Kawakami, Yoshiki Mikami
Obstet Gynecol Sci. 2025;68(1):79-89. doi: 10.5468/ogs.24236.
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013; 49:3262–73.
Article3. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64:444–53.
Article4. Safaeian M, Solomon D, Castle PE. Cervical cancer prevention--cervical screening: science in evolution. Obstet Gynecol Clin North Am. 2007; 34:739–60. ix
Article5. Schiffman M, Solomon D. Clinical practice. Cervical-cancer screening with human papillomavirus and cytologic cotesting. N Engl J Med. 2013; 369:2324–31.
Article6. Wright TC, Stoler MH, Behrens CM, Sharma A, Zhang G, Wright TL. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015; 136:189–97.
Article7. Kong TW, Kim M, Kim YH, Kim YB, Kim J, Kim JW, et al. High-risk human papillomavirus testing as a primary screening for cervical cancer: position statement by the Korean Society of Obstetrics and Gynecology and the Korean Society of Gynecologic Oncology. Obstet Gynecol Sci. 2020; 63:107.
Article8. Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020; 70:321–46.
Article9. Egemen D, Cheung LC, Chen X, Demarco M, Perkins RB, Kinney W, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020; 24:132–43.
Article10. Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020; 24:102–31.11. Schiffman M, Wentzensen N, Perkins RB, Guido RS. An introduction to the 2019 ASCCP risk-based management consensus guidelines. J Low Genit Tract Dis. 2020; 24:87–9.
Article12. ASCUS-LSIL Traige Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003; 188:1383–92.13. Solomon D, Schiffman M, Tarone R; ALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001; 93:293–9.
Article14. Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL triage study (ALTS). Arch Pathol Lab Med. 2003; 127:946–9.
Article15. Safaeian M, Solomon D, Wacholder S, Schiffman M, Castle P. Risk of precancer and follow-up management strategies for women with human papillomavirus-negative atypical squamous cells of undetermined significance. Obstet Gynecol. 2007; 109:1325–31.
Article16. The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst. 2000; 92:397–402.17. ASCUS-LSIL Traige Study (ALTS) Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol. 2003; 188:1393–400.18. Darragh TM, Colgan TJ, Cox JT, Heller DS, Henry MR, Luff RD, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis. 2012; 16:205–42.19. Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013; 17(5 Suppl 1):S1–27.
Article20. Kelly RS, Patnick J, Kitchener HC, Moss SM; NHSCSP HPV Special Interest Group. HPV testing as a triage for borderline or mild dyskaryosis on cervical cytology: results from the sentinel sites study. Br J Cancer. 2011; 105:983–8.
Article21. Silveira FA, Almeida G, Furtado Y, Silva KS, Maldonado P, Cavalcanti S, et al. HPV DNA genotyping and methylation of gene p16 INK4A in cervical LSIL. Exp Mol Pathol. 2015; 98:308–11.22. Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Five-year risks of CIN 2+ and CIN 3+ among women with HPV-positive and HPV-negative LSIL pap results. J Low Genit Tract Dis. 2013; 17(5 Suppl 1):S43–9.
Article23. Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013; 17(5 Suppl 1):S28–35.
Article24. Wang J, Tang D, Wang J, Zhang Z, Chen Y, Wang K, et al. Genotype distribution and prevalence of human papillomavirus among women with cervical cytological abnormalities in Xinjiang, China. Hum Vaccin Immunother. 2019; 15:1889–96.
Article25. Hwang HS, Park M, Lee SY, Kwon KH, Pang MG. Distribution and prevalence of human papillomavirus genotypes in routine Pap smear of 2,470 Korean women determined by DNA chip. Cancer Epidemiol Biomarkers Prev. 2004; 13:2153–6.
Article26. Ekalaksananan T, Pientong C, Kongyingyoes B, Chaiwongkot A, Yuenyao P, Kleebkaow P, et al. Combined p16INK4a and human papillomavirus testing improves the prediction of cervical intraepithelial neoplasia (CIN II–III) in Thai patients with low-grade cytological abnormalities. Asian Pac J Cancer Prev. 2011; 12:1777–83.27. Khunamornpong S, Settakorn J, Sukpan K, Srisomboon J, Suprasert P, Siriaunkgul S. Performance of HPV DNA testing with hybrid capture 2 in triaging women with minor cervical cytologic abnormalities (ASC-US/LSIL) in Northern Thailand. Asian Pac J Cancer Prev. 2014; 15:10961–6.
Article28. Siriaunkgul S, Settakorn J, Sukpan K, Srisomboon J, Suprasert P, Kasatpibal N, et al. Population-based cervical cancer screening using high-risk HPV DNA test and liquid-based cytology in northern Thailand. Asian Pac J Cancer Prev. 2014; 15:6837–42.
Article29. Phoolcharoen N, Kantathavorn N, Sricharunrat T, Saeloo S, Krongthong W. A population-based study of cervical cytology findings and human papillomavirus infection in a suburban area of Thailand. Gynecol Oncol Rep. 2017; 21:73–7.
Article30. Laowahutanont P, Karalak A, Wongsena M, Loonprom K, Pukcharoen P, Jamsri P, et al. Prevalence of high risk human papillomavirus infection with different cervical cytological features among women undergoing health examination at the National Cancer Institute, Thailand. Asian Pac J Cancer Prev. 2014; 15:5879–82.
Article31. Wright TC Jr, Stoler MH, Parvu V, Yanson K, Cooper C, Andrews J. Risk detection for high-grade cervical disease using onclarity HPV extended genotyping in women, ≥21 years of age, with ASC-US or LSIL cytology. Gynecol Oncol. 2019; 154:360–7.32. Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010; 202:1789–99.
Article33. Santos AL, Derchain SF, Martins MR, Sarian LO, Martinez EZ, Syrjänen KJ. Human papillomavirus viral load in predicting high-grade CIN in women with cervical smears showing only atypical squamous cells or low-grade squamous intraepithelial lesion. Sao Paulo Med J. 2003; 121:238–43.
Article34. Massad LS, Markwell S. Using patient history to predict high-grade cervical dysplasia and cancer in women with borderline cervical cytologic results. J Low Genit Tract Dis. 2004; 8:207–11.
Article35. Khuakoonratt N, Tangjitgamol S, Manusirivithaya S, Khunnarong J, Pataradule K, Thavaramara T, et al. Prevalence of high grade squamous intraepithelial lesion (HSIL) and invasive cervical cancer in patients with low grade squamous intraepithelial lesion (LSIL) at cervical pap smear. Asian Pac J Cancer Prev. 2008; 9:253–7.36. Carreon JD, Sherman ME, Guillén D, Solomon D, Herrero R, Jerónimo J, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol. 2007; 26:441–6.37. Tainio K, Athanasiou A, Tikkinen KAO, Aaltonen R, Cárdenas J, Hernándes , et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ. 2018; 360:k499.
Article38. Castle PE, Adcock R, Cuzick J, Wentzensen N, Torrez-Martinez NE, Torres SM, et al. Relationships of p16 immunohistochemistry and other biomarkers with diagnoses of cervical abnormalities: implications for LAST terminology. Arch Pathol Lab Med. 2020; 144:725–34.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of human papillomavirus genotype on severity and prognosis of cervical intraepithelial neoplasia
- Detection of Human Papillomavius DNA by Hybrid Capture Test in Cervical Intraepithelial Neoplasia and Carcinoma
- Visual inspection with acetic acid for detection of high grade lesion in atypical squamous cells and low grade squamous intraepithelial lesions from cervical Pap smear
- Human Papillomavirus Genotype Distribution Among 18,815 Women in 13 Korean Cities and Relationship With Cervical Cytology Findings
- Prevalence and Genotype of Human Papillomavirus Infection and Risk of Cervical Dysplasia among Asymptomatic Korean Women