Neurointervention.
2022 Jul;17(2):93-99. 10.5469/neuroint.2022.00129.
Reversible Symptom Aggravation by Intake of Taurine-Rich Foods in Patients with Venous Congestive Myelopathy: Controlled Case Series Study
- Affiliations
-
- 1Neurointervention Clinic, Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Neurointervention, GangNam St. Peter’s Hospital, Seoul, Korea
- 3Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2531564
- DOI: http://doi.org/10.5469/neuroint.2022.00129
Abstract
- Purpose
Reversible aggravation of myelopathy symptoms was observed after the intake of taurine-rich foods in patients with venous congestive myelopathy (VCM) caused by a spinal arteriovenous shunt (SAVS), and the taurine-challenge test was applied to demonstrate an association between taurine and VCM.
Materials and Methods
The current study reviewed any aggravation history of myelopathy symptoms, including walking difficulty, after consuming taurine-rich foods among 133 consecutive patients with a SAVS from a prospective institutional database from June 2013 to February 2021. The type of taurine-rich foods, demographic data, arteriovenous shunt level, and follow-up periods were obtained. For the controlled taurine challenge test, Bacchus® (Dong-A Pharmaceutical, Seoul, Korea), a taurine-rich drink, was given to patients who fulfilled test criteria of recovered VCM (pain-sensory-motor-sphincter scale ≥2, improvement of spinal cord signal intensity on magnetic resonance imaging, and follow-up >6 months after SAVS treatment) to confirm the disappearance of such aggravation.
Results
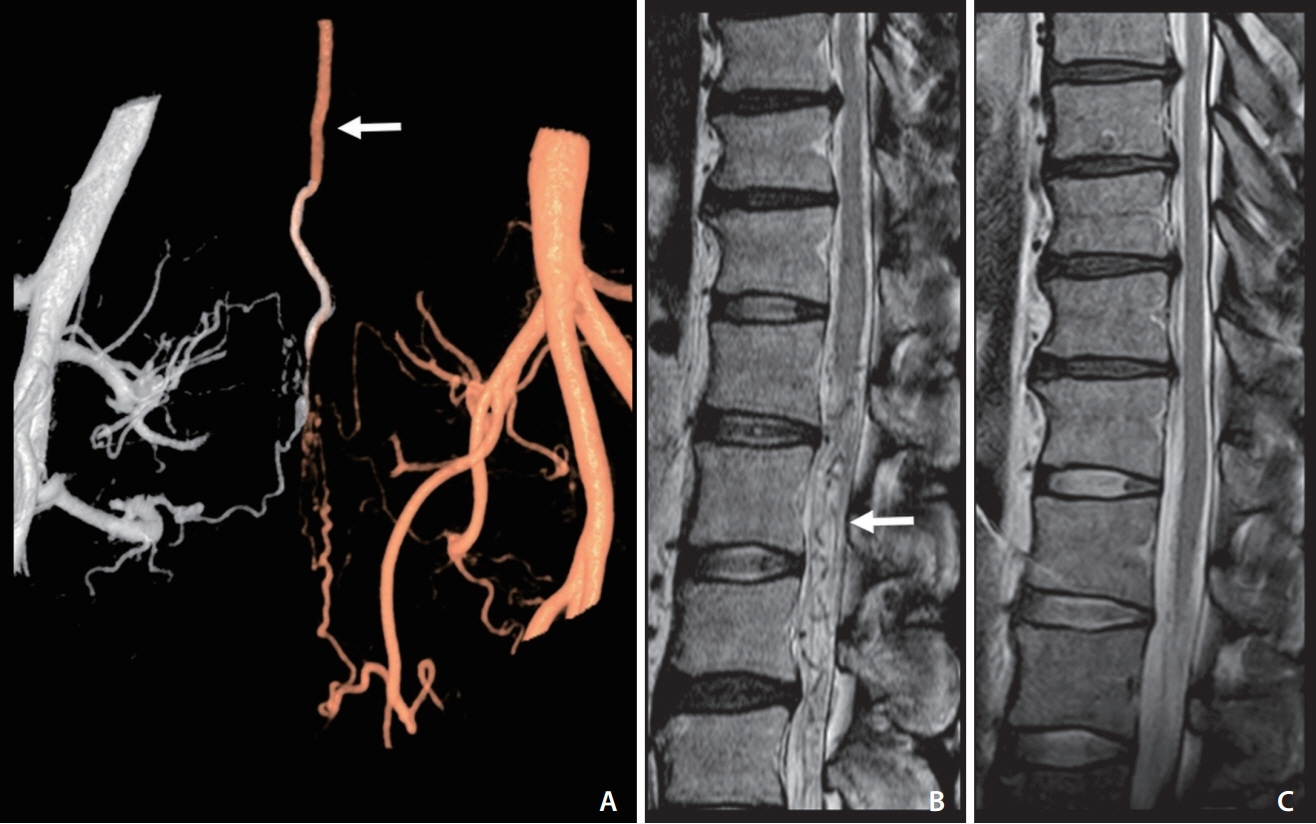
Ten patients had an aggravation history related to food. Webfoot octopus, small octopus, squid, crab, scallop, and taurine-rich energy drink (Bacchus®) were related to such aggravation in patients with VCM. Aggravation appeared about 30 minutes after food intake followed by expressions such as ‘I could not walk and collapsed to the ground’ and usually lasted for about 3 hours, followed by a slow recovery after taking rest. Four patients who met the test criteria underwent the taurine challenge with Bacchus® and revealed no further symptom aggravation, suggesting that taurine did not affect patients after recovery from VCM.
Conclusion
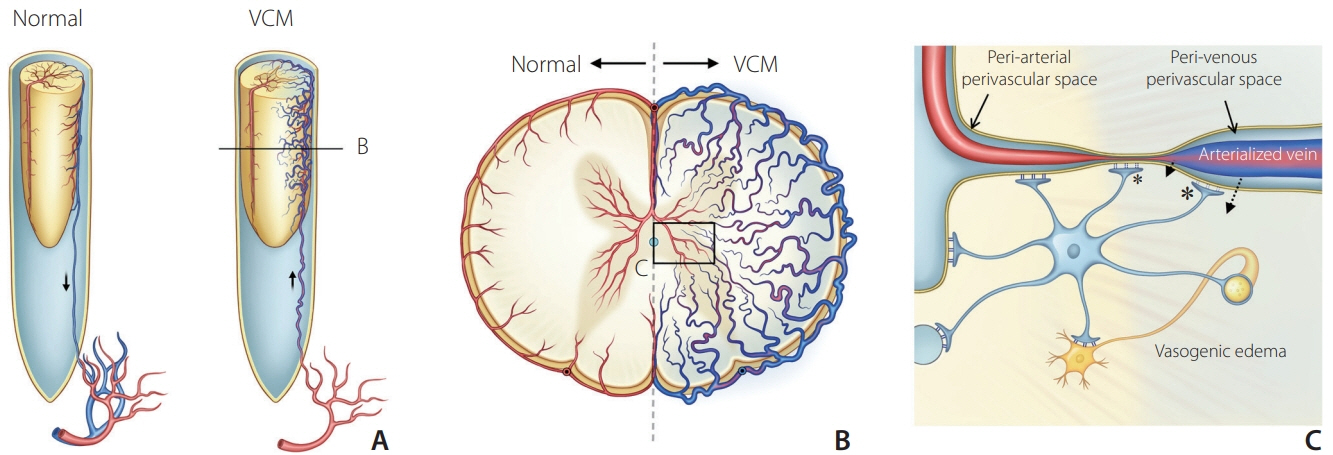
The association between taurine-rich food and reversible symptom aggravation can appear in patients with VCM and disappear after VCM treatment. Aggravation of venous hypertension in the spinal cord is suggested as a mechanism but further elucidation is needed.
Keyword
Figure
Cited by 1 articles
-
Venous Congestive Myelopathy Caused by Spinal Vascular Malformation
Dae Chul Suh
Neurointervention. 2023;18(2):77-79. doi: 10.5469/neuroint.2023.00262.
Reference
-
1. Lee CS, Pyun HW, Chae EY, Kim KK, Rhim SC, Suh DC. Reversible aggravation of neurological deficits after steroid medication in patients with venous congestive myelopathy caused by spinal arteriovenous malformation. Interv Neuroradiol. 2009; 15:325–329.
Article2. Suh DC, Song Y, Park D, Han M, Lim YM, Park JE, et al. New grading system for the clinical evaluation of patients with spinal vascular lesions. Neuroradiology. 2018; 60:1035–1041.
Article3. Rafiee Z, García-Serrano AM, Duarte JMN. Taurine supplementation as a neuroprotective strategy upon brain dysfunction in metabolic syndrome and diabetes. Nutrients. 2022; 14:1292.
Article4. Gutiérrez-Hellín J, Varillas-Delgado D. Energy drinks and sports performance, cardiovascular risk, and genetic associations; future prospects. Nutrients. 2021; 13:715.
Article5. Kim ES, Kim JS, Moon HK. Taurine contents in commercial milks, meats and seafoods. J Korean Soc Food Sci Nutr. 1999; 28:16–21.6. Rutherford JA, Spriet LL, Stellingwerff T. The effect of acute taurine ingestion on endurance performance and metabolism in well-trained cyclists. Int J Sport Nutr Exerc Metab. 2010; 20:322–329.
Article7. Cho TA, Bhattacharyya S. Approach to myelopathy. Continuum (Minneap Minn). 2018; 24:386–406.
Article8. Rabinstein AA. Vascular myelopathies. Continuum (Minneap Minn). 2015; 21:67–83.
Article9. Muralidharan R, Saladino A, Lanzino G, Atkinson JL, Rabinstein AA. The clinical and radiological presentation of spinal dural arteriovenous fistula. Spine (Phila Pa 1976). 2011; 36:E1641–E1647.
Article10. Adrianto Y, Yang KH, Koo HW, Park W, Jung SC, Park JE, et al. Concomitant origin of the anterior or posterior spinal artery with the feeder of a spinal dural arteriovenous fistula (SDAVF). J Neurointerv Surg. 2017; 9:405–410.
Article11. Chul Suh D, Gon Choi C, Bo Sung K, Kim KK, Chul Rhim S. Spinal osseous epidural arteriovenous fistula with multiple small arterial feeders converging to a round fistular nidus as a target of venous approach. AJNR Am J Neuroradiol. 2004; 25:69–73.12. Jung SC, Song Y, Cho SH, Kim J, Noh SY, Lee SH, et al. Endovascular management of aneurysms associated with spinal arteriovenous malformations. J Neurointerv Surg. 2018; 10:198–203.
Article13. Park JE, Koo HW, Liu H, Jung SC, Park D, Suh DC. Clinical characteristics and treatment outcomes of spinal arteriovenous malformations. Clin Neuroradiol. 2018; 28:39–46.
Article14. Song Y, Cho SH, Lee DW, Sheen JJ, Shin JH, Suh DC. Osseous versus nonosseous spinal epidural arteriovenous fistulas: experiences of 13 patients. AJNR Am J Neuroradiol. 2019; 40:129–134.
Article15. Suh DC, Cho SH, Park JE, Liu H, Jung SC. Induced-wedge technique to improve liquid embolic agent penetration into spinal dural arteriovenous fistula. World Neurosurg. 2016; 96:309–315.
Article16. Suh DC, Kim HS, Baek HJ, Park JW, Kim KK, Rhim SC. Angioarchitecture of spinal dural arteriovenous fistula - evaluation with 3D rotational angiography. Neurointervention. 2012; 7:10–16.
Article17. Grasser EK, Yepuri G, Dulloo AG, Montani JP. Cardio- and cerebrovascular responses to the energy drink Red Bull in young adults: a randomized cross-over study. Eur J Nutr. 2014; 53:1561–1571.
Article18. Nycz B, Mandera M. The features of the glymphatic system. Auton Neurosci. 2021; 232:102774.
Article19. Kaur J, Fahmy LM, Davoodi-Bojd E, Zhang L, Ding G, Hu J, et al. Waste clearance in the brain. Front Neuroanat. 2021; 15:665803.
Article20. Bakhsheshian J, Strickland BA, Mack WJ, Zlokovic BV. Investigating the blood-spinal cord barrier in preclinical models: a systematic review of in vivo imaging techniques. Spinal Cord. 2021; 59:596–612.
Article21. Zalewski NL, Rabinstein AA, Brinjikji W, Kaufmann TJ, Nasr D, Ruff MW, et al. Unique gadolinium enhancement pattern in spinal dural arteriovenous fistulas. JAMA Neurol. 2018; 75:1542–1545.
Article22. Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2009; 30:639–648.
Article23. Schaffer S, Kim HW. Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther (Seoul). 2018; 26:225–241.
Article24. Chupel MU, Minuzzi LG, Furtado G, Santos ML, Hogervorst E, Filaire E, et al. Exercise and taurine in inflammation, cognition, and peripheral markers of blood-brain barrier integrity in older women. Appl Physiol Nutr Metab. 2018; 43:733–741.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects and Mechanisms of Taurine as a Therapeutic Agent
- Congestive myelopathy due to spinal dural arteriovenous fistula mimicking CNS demyelinating disease
- Venous Congestive Myelopathy Caused by Spinal Vascular Malformation
- A case of taurine-containing drink induced anaphylaxis
- Effects of dietary taurine supplementation on plasma and liver lipids in OVX rats fed calcium-deficient diet