Cancer Res Treat.
2022 Jul;54(3):850-859. 10.4143/crt.2021.674.
Radiofrequency Ablation versus Stereotactic Body Radiation Therapy in the Treatment of Colorectal Cancer Liver Metastases
- Affiliations
-
- 1Department of Radiation Oncology, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 2Department of Radiology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Department of Colon and Rectal Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2531331
- DOI: http://doi.org/10.4143/crt.2021.674
Abstract
- Purpose
This study aimed to compare the treatment outcomes of radiofrequency ablation (RFA) and stereotactic body radiation therapy (SBRT) for colorectal cancer liver metastases (CRLM) and to determine the favorable treatment modality according to tumor characteristics.
Materials and Methods
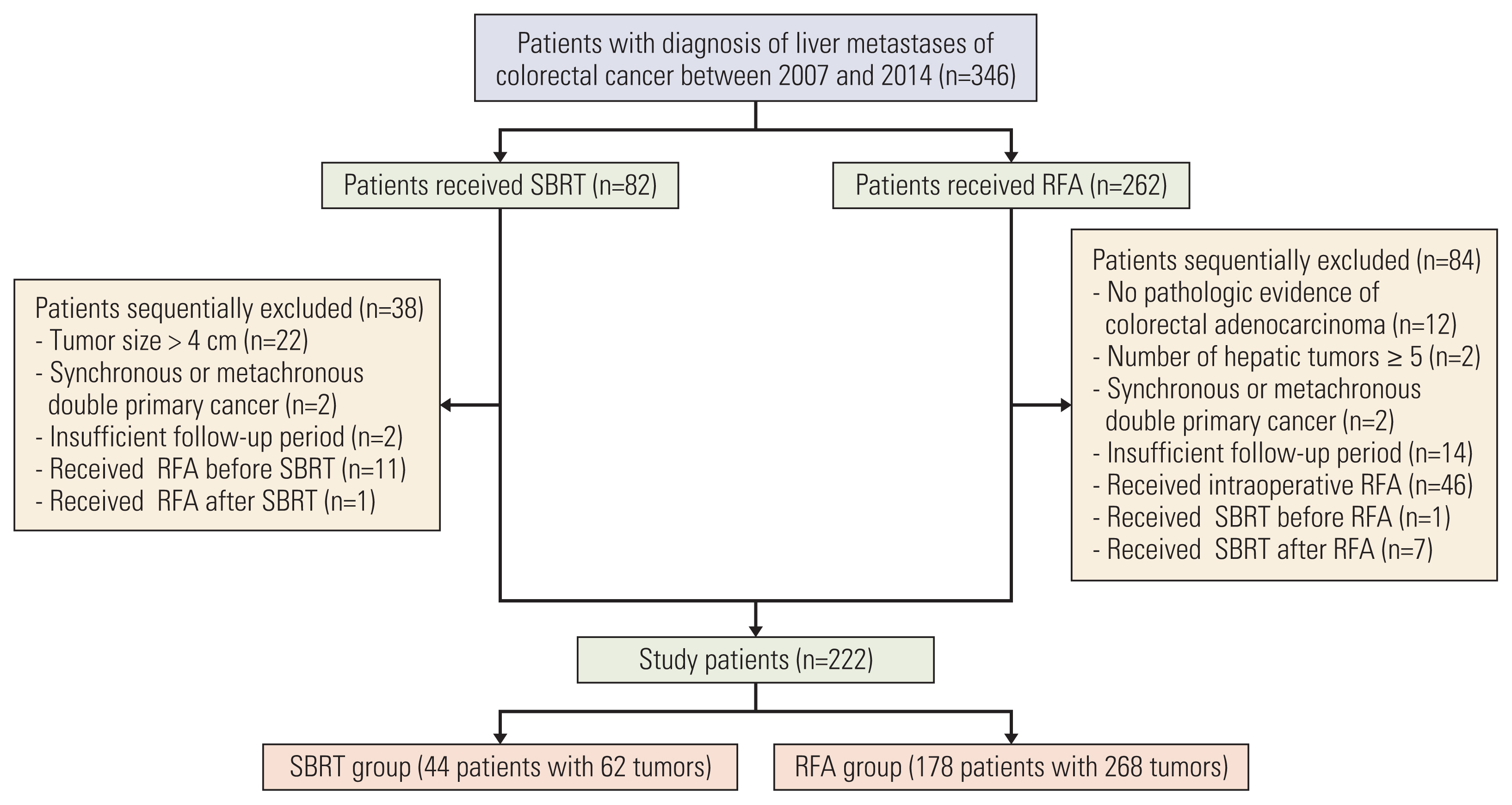
We retrospectively analyzed the records of 222 colorectal cancer patients with 330 CRLM who underwent RFA (268 tumors in 178 patients) or SBRT (62 tumors in 44 patients) between 2007 and 2014. Kaplan–Meier method and Cox models were used by adjusting with inverse probability of treatment weighting (IPTW).
Results
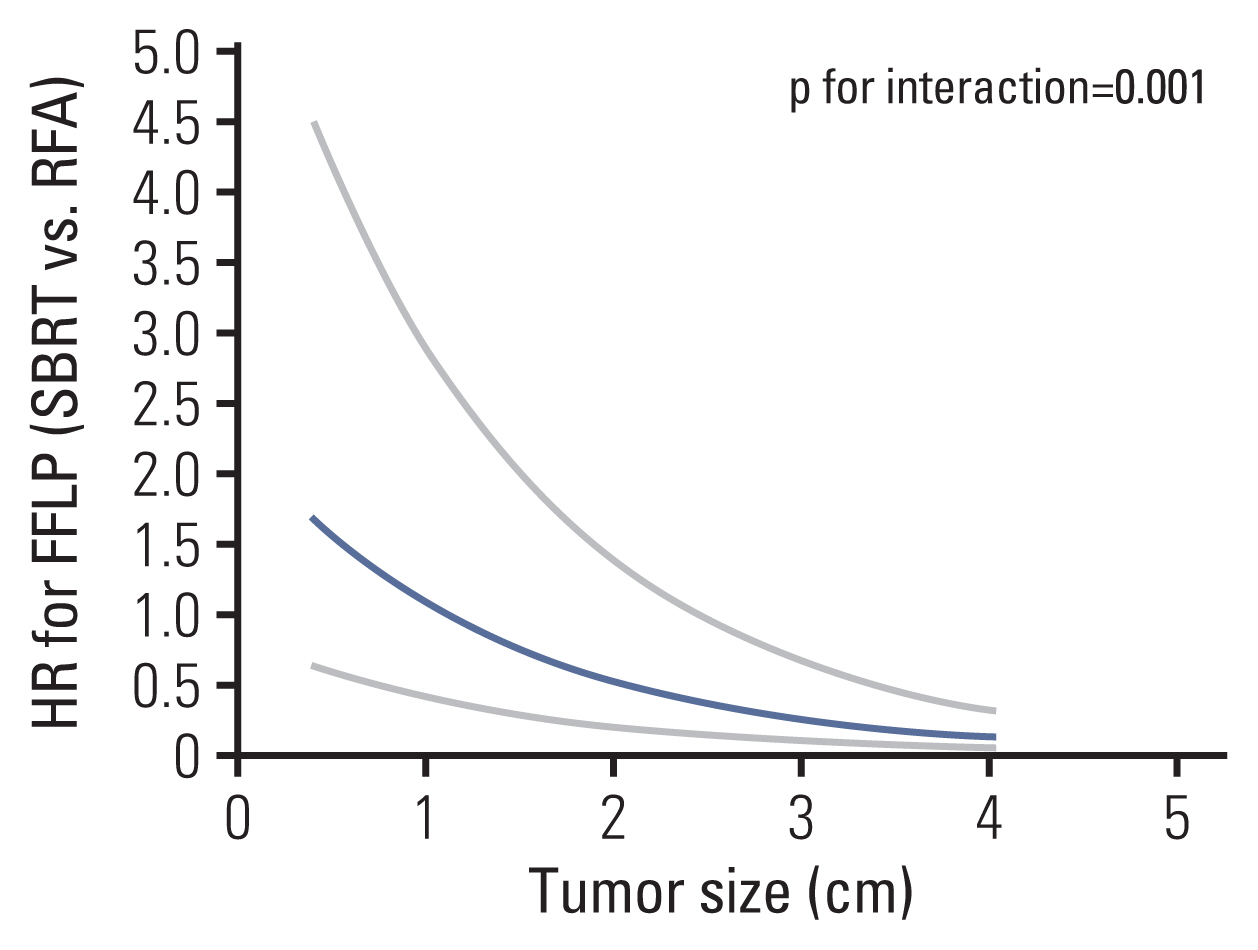
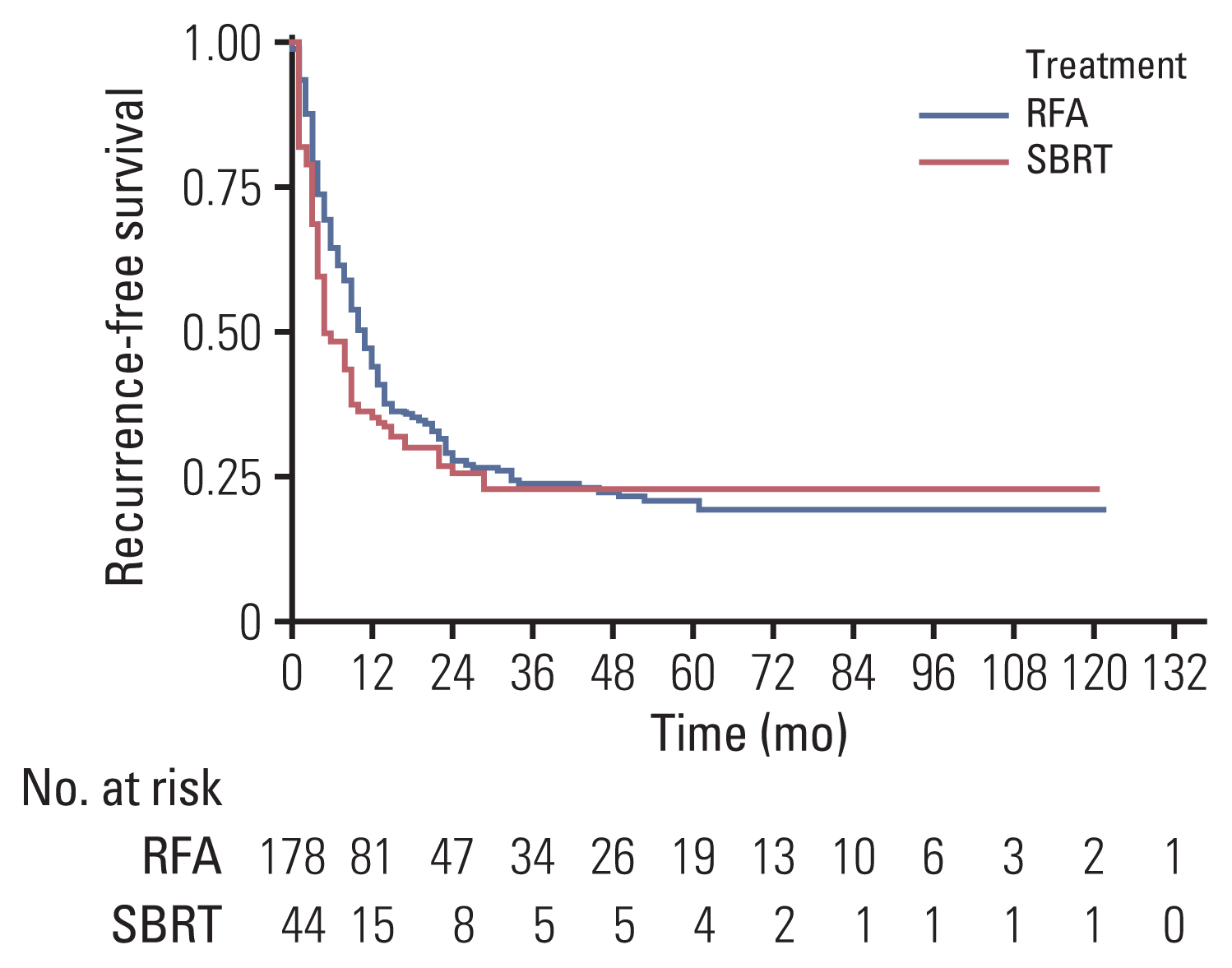
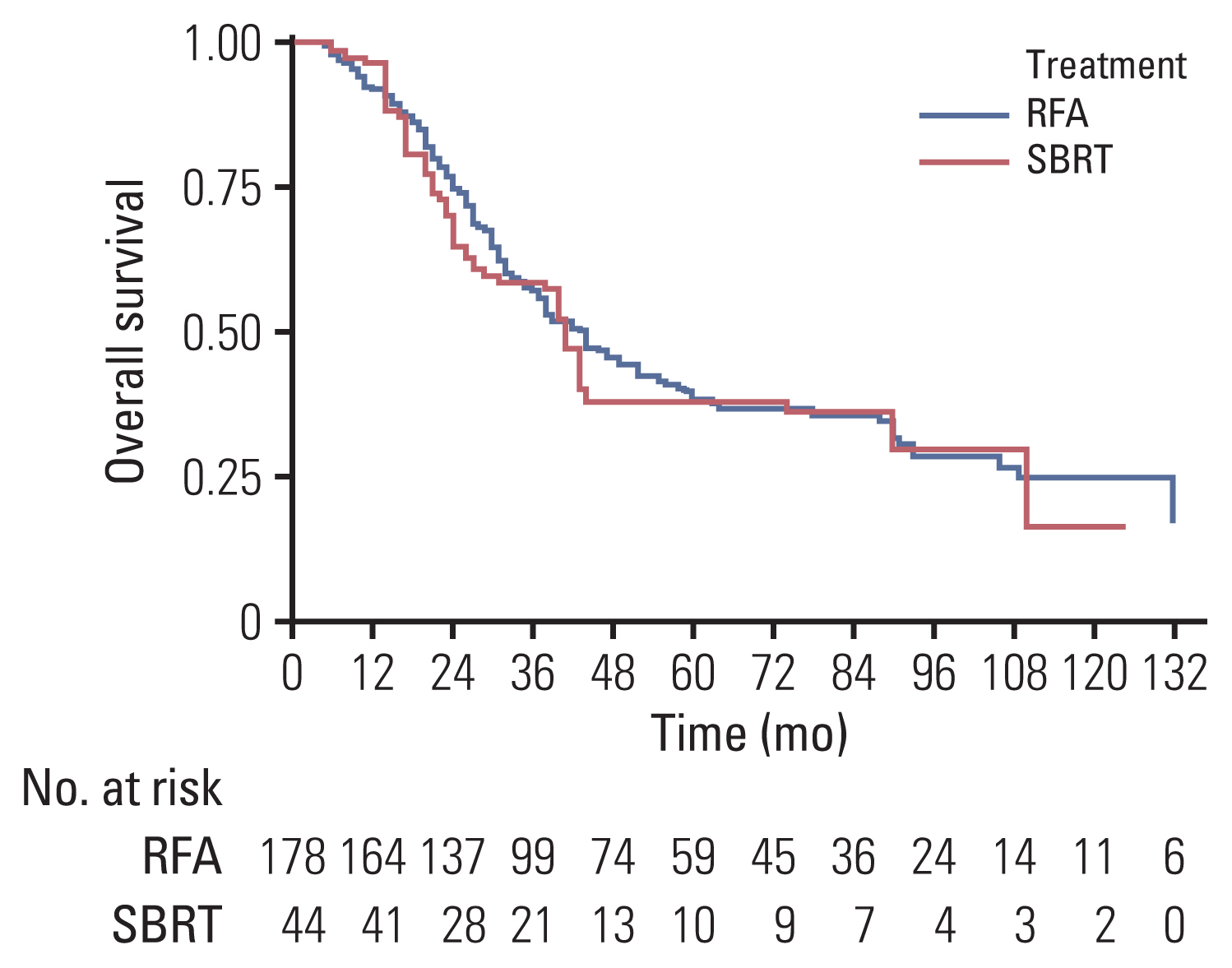
The median follow-up duration was 30.5 months. The median tumor size was significantly smaller in the RFA group than in the SBRT group (1.5 cm vs 2.3 cm, p<0.001). In IPTW-adjusted analysis, difference in treatment modality was not associated with significant differences in 1-year and 3-year recurrence-free survival (35% vs 43%, 22% vs 23%; p=0.198), overall survival (96% vs 91%, 58% vs 56%; p=0.508), and freedom from local progression (FFLP; 90% vs 72%, 78% vs 60%; p=0.106). Significant interaction effect between the treatment modality and tumor size was observed for FFLP (p=0.001). In IPTW-adjusted subgroup analysis of patients with tumor size >2 cm, the SBRT group had a higher FFLP compared with the RFA group (HR, 0.153; p<0.001).
Conclusion
SBRT and RFA showed similar local control in the treatment of patients with CRLM. Tumor size was an independent prognostic factor for local control and SBRT may be preferred for larger tumors.
Keyword
Figure
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer [Internet]. Bethesda, MD: National Cancer Institute;2021. [cited 2021 Feb 2]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html .3. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014; 14:810.
Article4. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009; 27:3677–83.
Article5. Venkat SR, Mohan PP, Gandhi RT. Colorectal liver metastasis: overview of treatment paradigm highlighting the role of ablation. AJR Am J Roentgenol. 2018; 210:883–90.
Article6. Chang DT, Swaminath A, Kozak M, Weintraub J, Koong AC, Kim J, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011; 117:4060–9.
Article7. Scorsetti M, Comito T, Tozzi A, Navarria P, Fogliata A, Clerici E, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol. 2015; 141:543–53.
Article8. Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006; 45:823–30.
Article9. Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD 3rd, Dorfman GS, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010; 28:493–508.
Article10. Ruers T, Van Coevorden F, Punt CJ, Pierie JE, Borel-Rinkes I, Ledermann JA, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase II trial. J Natl Cancer Inst. 2017; 109:djx015.
Article11. Tanis E, Nordlinger B, Mauer M, Sorbye H, van Coevorden F, Gruenberger T, et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases: analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer. 2014; 50:912–9.
Article12. Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005; 242:158–71.13. Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006; 43:1101–8.
Article14. Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, et al. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003; 97:1253–62.
Article15. Joo JH, Park JH, Kim JC, Yu CS, Lim SB, Park IJ, et al. Local control outcomes using stereotactic body radiation therapy for liver metastases from colorectal cancer. Int J Radiat Oncol Biol Phys. 2017; 99:876–83.
Article16. Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009; 27:1572–8.
Article17. Jackson WC, Tao Y, Mendiratta-Lala M, Bazzi L, Wahl DR, Schipper MJ, et al. Comparison of stereotactic body radiation therapy and radiofrequency ablation in the treatment of intrahepatic metastases. Int J Radiat Oncol Biol Phys. 2018; 100:950–8.
Article18. Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016; 34:452–9.
Article19. Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010; 33:11–7.
Article20. Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010; 78(Suppl 1):113–24.
Article21. Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007; 10:38–46.
Article22. McPartlin A, Swaminath A, Wang R, Pintilie M, Brierley J, Kim J, et al. Long-term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int J Radiat Oncol Biol Phys. 2017; 99:388–95.
Article23. van der Pool AE, Mendez Romero A, Wunderink W, Heijmen BJ, Levendag PC, Verhoef C, et al. Stereotactic body radiation therapy for colorectal liver metastases. Br J Surg. 2010; 97:377–82.
Article24. Klement RJ, Guckenberger M, Alheid H, Allgauer M, Becker G, Blanck O, et al. Stereotactic body radiotherapy for oligometastatic liver disease: influence of pre-treatment chemotherapy and histology on local tumor control. Radiother Oncol. 2017; 123:227–33.25. Klement RJ. Radiobiological parameters of liver and lung metastases derived from tumor control data of 3719 metastases. Radiother Oncol. 2017; 123:218–26.
Article26. Yang G, Wang G, Sun J, Xiong Y, Li W, Tang T, et al. The prognosis of radiofrequency ablation versus hepatic resection for patients with colorectal liver metastases: a systematic review and meta-analysis based on 22 studies. Int J Surg. 2021; 87:105896.
Article27. Kim N, Cheng J, Huang WY, Kimura T, Zeng ZC, Lee VH, et al. Dose-response relationship in stereotactic body radiation therapy for hepatocellular carcinoma: a pooled analysis of an Asian Liver Radiation Therapy Group Study. Int J Radiat Oncol Biol Phys. 2021; 109:464–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiofrequency Ablation of Liver Metastases from Colorectal Cancer: A Literature Review
- Treatment of Liver Metastases
- Liver Metastases in Colorectal Cancer
- Huge Hepatocellular Carcinoma Exhibiting a Complete Response after Stereotactic Body Radiation Therapy
- Management of Colorectal Cancer Liver Metastasis