Ann Pediatr Endocrinol Metab.
2022 Jun;27(2):90-97. 10.6065/apem.2244114.057.
Clinical management and emerging therapies of FGFR3-related skeletal dysplasia in childhood
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Children’s Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Rare Disease Center, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2531272
- DOI: http://doi.org/10.6065/apem.2244114.057
Abstract
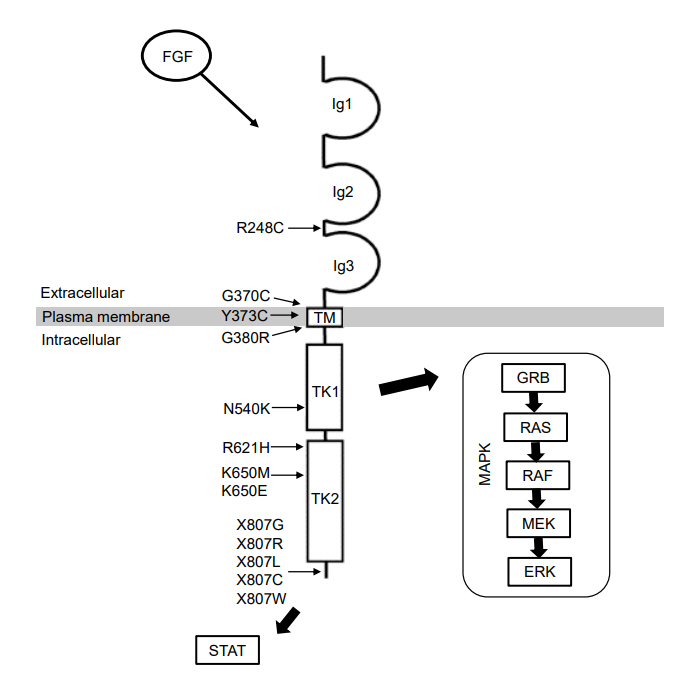
- Skeletal dysplasia is a diverse group of disorders that affect bone development and morphology. Currently, approximately 461 different genetic skeletal disorders have been identified, with over 430 causative genes. Among these, fibroblast growth factor receptor 3 (FGFR3)-related skeletal dysplasia is a relatively common subgroup of skeletal dysplasia. Pediatric endocrinologists may encounter a suspected case of skeletal dysplasia in their practice, especially when evaluating children with short stature. Early and accurate diagnosis of FGFR3-related skeletal dysplasia is essential for timely management of complications and genetic counseling. This review summarizes 5 representative and distinct entities of skeletal dysplasia caused by pathogenic variants in FGFR3 and discusses emerging therapies for FGFR3-related skeletal dysplasias.
Keyword
Figure
Reference
-
References
1. Du X, Xie Y, Xian CJ, Chen L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J Cell Physiol. 2012; 227:3731–43.2. Foldynova-Trantirkova S, Wilcox WR, Krejci P. Sixteen years and counting: the current understanding of fibroblast growth factor receptor 3 (FGFR3) signaling in skeletal dysplasias. Hum Mutat. 2012; 33:29–41.3. Toydemir RM, Brassington AE, Bayrak-Toydemir P, Krakowiak PA, Jorde LB, Whitby FG, et al. A novel mutation in FGFR3 causes camptodactyly, tall stature, and hearing loss (CATSHL) syndrome. Am J Hum Genet. 2006; 79:935–41.4. Makrythanasis P, Temtamy S, Aglan MS, Otaify GA, Hamamy H, Antonarakis SE. A novel homozygous mutation in FGFR3 causes tall stature, severe lateral tibial deviation, scoliosis, hearing impairment, camptodactyly, and arachnodactyly. Hum Mutat. 2014; 35:959–63.5. Sargar KM, Singh AK, Kao SC. Imaging of skeletal disorders caused by fibroblast growth factor receptor gene mutations. Radiographics. 2017; 37:1813–30.6. Marzin P, Cormier-Daire V. New perspectives on the treatment of skeletal dysplasia. Ther Adv Endocrinol Metab. 2020; 11:2042018820904016.7. Legeai-Mallet L, Savarirayan R. Novel therapeutic approaches for the treatment of achondroplasia. Bone. 2020; 141:115579.8. Laederich MB, Horton WA. Achondroplasia: pathogenesis and implications for future treatment. Curr Opin Pediatr. 2010; 22:516–23.9. Narayana J, Horton WA. FGFR3 biology and skeletal disease. Connect Tissue Res. 2015; 56:427–33.10. Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996; 84:911–21.11. Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005; 16:139–49.12. Segev O, Chumakov I, Nevo Z, Givol D, Madar-Shapiro L, Sheinin Y, et al. Restrained chondrocyte proliferation and maturation with abnormal growth plate vascularization and ossification in human FGFR-3(G380R) transgenic mice. Hum Mol Genet. 2000; 9:249–58.13. Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996; 12:390–7.14. Escobar LF, Tucker M, Bamshad M. A second family with CATSHL syndrome: confirmator y report of another unique FGFR3 syndrome. Am J Med Genet A. 2016; 170:1908–11.15. Offiah AC, Hall CM. Radiological diagnosis of the constitutional disorders of bone. As easy as A, B, C? Pediatr Radiol. 2003; 33:153–61.16. Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007; 370:162–72.17. Waller DK, Correa A, Vo TM, Wang Y, Hobbs C, Langlois PH, et al. The population-based prevalence of achondroplasia and thanatophoric dysplasia in selected regions of the US. Am J Med Genet A. 2008; 146A:2385–9.18. Orioli IM, Castilla EE, Scarano G, Mastroiacovo P. Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. Am J Med Genet. 1995; 59:209–17.19. Bellus GA, Hefferon TW, Ortiz de Luna RI, Hecht JT, Horton WA, Machado M, et al. Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet. 1995; 56:368–73.20. Legare JM. Achondroplasia. In : Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, editors. GeneReviews((R)). Seattle (WA): University of Washington, Seattle;1993-2022.21. Kubota T, Adachi M, Kitaoka T, Hasegawa K, Ohata Y, Fujiwara M, et al. Clinical practice guidelines for achondroplasia. Clin Pediatr Endocrinol. 2020; 29:25–42.22. Hoover-Fong J, Scott CI, Jones MC; COMMITTEE ON GENETICS. Health supervision for people with achondroplasia. Pediatrics. 2020; 145:e20201010.23. Horton WA, Rotter JI, Rimoin DL, Scott CI, Hall JG. Standard growth curves for achondroplasia. J Pediatr. 1978; 93:435–8.24. Hecht JT, Francomano CA, Horton WA, Annegers JF. Mortality in achondroplasia. Am J Hum Genet. 1987; 41:454–64.25. Panda A, Gamanagatti S, Jana M, Gupta AK. Skeletal dysplasias: a radiographic approach and review of common non-lethal skeletal dysplasias. World J Radiol. 2014; 6:808–25.26. McKusick VA, Kelly TE, Dorst JP. Observations suggesting allelism of the achondroplasia and hypochondroplasia genes. J Med Genet. 1973; 10:11–6.27. Prinos P, Costa T, Sommer A, Kilpatrick MW, Tsipouras P. A common FGFR3 gene mutation in hypochondroplasia. Hum Mol Genet. 1995; 4:2097–101.28. Rousseau F, Bonaventure J, Legeai-Mallet L, Schmidt H, Weissenbach J, Maroteaux P, et al. Clinical and genetic heterogeneity of hypochondroplasia. J Med Genet. 1996; 33:749–52.29. Xue Y, Sun A, Mekikian PB, Martin J, Rimoin DL, Lachman RS, et al. FGFR3 mutation frequency in 324 cases from the International Skeletal Dysplasia Registry. Mol Genet Genomic Med. 2014; 2:497–503.30. Glasgow JF, Nevin NC, Thomas PS. Hypochondroplasia. Arch Dis Child. 1978; 53:868–72.31. Arenas MA, Del Pino M, Fano V. FGFR3-related hypochondroplasia: longitudinal growth in 57 children with the p.Asn540Lys mutation. J Pediatr Endocrinol Metab. 2018; 31:1279–84.32. Wynne-Davies R, Walsh WK, Gormley J. Achondroplasia and hypochondroplasia. Clinical variation and spinal stenosis. J Bone Joint Surg Br. 1981; 63B:508–15.33. Wynne-Davies R, Patton MA. The frequency of mental retardation in hypochondroplasia. J Med Genet. 1991; 28:644.34. Linnankivi T, Makitie O, Valanne L, Toiviainen-Salo S. Neuroimaging and neurological findings in patients with hypochondroplasia and FGFR3 N540K mutation. Am J Med Genet A. 2012; 158A:3119–25.35. Orioli IM, Castilla EE, Barbosa-Neto JG. The birth prevalence rates for the skeletal dysplasias. J Med Genet. 1986; 23:328–32.36. Donnelly DE, McConnell V, Paterson A, Morrison PJ. The prevalence of thanatophoric dysplasia and lethal osteogenesis imperfecta type II in Northern Ireland - a complete population study. Ulster Med J. 2010; 79:114–8.37. French T, Savarirayan R. Thanatophoric dysplasia. 2004 May 21 [updated 2020 Jun 18. In : Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle;1993-2022.38. Hurst JA, Firth HV, Smithson S. Skeletal dysplasias. Semin Fetal Neonatal Med. 2005; 10:233–41.39. MacDonald IM, Hunter AG, MacLeod PM, MacMurray SB. Growth and development in thanatophoric dysplasia. Am J Med Genet. 1989; 33:508–12.40. Nikkel SM, Major N, King WJ. Growth and development in thanatophoric dysplasia - an update 25 years later. Clin Case Rep. 2013; 1:75–8.41. Baker KM, Olson DS, Harding CO, Pauli RM. Long-term survival in typical thanatophoric dysplasia type 1. Am J Med Genet. 1997; 70:427–36.42. Nakai K, Yoneda K, Moriue T, Munehiro A, Fujita N, Moriue J, et al. Seborrhoeic keratoses and acanthosis nigricans in a long-term survivor of thanatophoric dysplasia. Br J Dermatol. 2010; 163:656–8.43. Katsumata N, Kuno T, Miyazaki S, Mikami S, Nagashima-Miyokawa A, Nimura A, et al. G370C mutation in the FGFR3 gene in a Japanese patient with thanatophoric dysplasia. Endocr J. 1998; 45 Suppl:S171–4.44. Kuno T, Fujita I, Miyazaki S, Katsumata N. Markers for bone metabolism in a long-lived case of thanatophoric dysplasia. Endocr J. 2000; 47 Suppl:S141–4.45. Tavormina PL, Bellus GA, Webster MK, Bamshad MJ, Fraley AE, McIntosh I, et al. A novel skeletal dysplasia with developmental delay and acanthosis nigricans is caused by a Lys650Met mutation in the fibroblast growth factor receptor 3 gene. Am J Hum Genet. 1999; 64:722–31.46. Zankl A, Elakis G, Susman RD, Inglis G, Gardener G, Buckley MF, et al. Prenatal and postnatal presentation of severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN) due to the FGFR3 Lys650Met mutation. Am J Med Genet A. 2008; 146A:212–8.47. Bellus GA, Bamshad MJ, Przylepa KA, Dorst J, Lee RR, Hurko O, et al. Severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN): phenotypic analysis of a new skeletal dysplasia caused by a Lys650Met mutation in fibroblast growth factor receptor 3. Am J Med Genet. 1999; 85:53–65.48. Harada D, Namba N, Hanioka Y, Ueyama K, Sakamoto N, Nakano Y, et al. Final adult height in long-term growth hormone-treated achondroplasia patients. Eur J Pediatr. 2017; 176:873–9.49. Massart F, Miccoli M, Baggiani A, Bertelloni S. Height outcome of short children with hypochondroplasia after recombinant human growth hormone treatment: a meta-analysis. Pharmacogenomics. 2015; 16:1965–73.50. Ko KR, Shim JS, Chung CH, Kim JH. Surgical results of limb lengthening at the femur, tibia, and humerus in patients with achondroplasia. Clin Orthop Surg. 2019; 11:226–32.51. Al Shaer D, Al Musaimi O, Albericio F, de la Torre BG. 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals (Basel). 2022; 15:222.52. Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X, et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet. 1999; 23:18–20.53. Krejci P, Masri B, Fontaine V, Mekikian PB, Weis M, Prats H, et al. Interaction of fibroblast growth factor and C-natriuretic peptide signaling in regulation of chondrocyte proliferation and extracellular matrix homeostasis. J Cell Sci. 2005; 118:5089–100.54. Yasoda A, Komatsu Y, Chusho H, Miyazawa T, Ozasa A, Miura M, et al. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004; 10:80–6.55. Suhasini M, Li H, Lohmann SM, Boss GR, Pilz RB. Cyclic-GMP-dependent protein kinase inhibits the Ras/Mitogen-activated protein kinase pathway. Mol Cell Biol. 1998; 18:6983–94.56. Savarirayan R, Tofts L, Irving M, Wilcox W, Bacino CA, Hoover-Fong J, et al. Once-daily, subcutaneous vosoritide therapy in children with achondroplasia: a randomised, double-blind, phase 3, placebo-controlled, multicentre trial. Lancet. 2020; 396:684–92.57. Unger S, Bonafe L, Gouze E. Current care and investigational therapies in achondroplasia. Curr Osteoporos Rep. 2017; 15:53–60.58. Matsushita M, Hasegawa S, Kitoh H, Mori K, Ohkawara B, Yasoda A, et al. Meclozine promotes longitudinal skeletal growth in transgenic mice with achondroplasia carrying a gain-of-function mutation in the FGFR3 gene. Endocrinology. 2015; 156:548–54.59. Yamashita A, Morioka M, Kishi H, Kimura T, Yahara Y, Okada M, et al. Statin treatment rescues FGFR3 skeletal dysplasia phenotypes. Nature. 2014; 513:507–11.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mutation at exon 10 of the fibroblast growth factor receptor 3 (FGFR3) in a fetus with thanatophoric dysplasia type I (TDI)
- Mutation analysis of Fibroblast Growth Factor Receptor 3 (FGFR3) Gene in Korean Patients with Achondroplasia and Hypochondroplasia
- A Case of Acanthosis Nigricans with Hypochondroplasia due to FGFR3 Gene (p.Lys650Thr) Mutation
- A case of thanatophoric dysplasia type I with an R248C mutation in the FGFR3 gene
- Thanatophoric dysplasia in a dichorionic twin confirmed by genetic analysis at the early second trimester: A case report and literature review