J Korean Med Sci.
2022 Jul;37(27):e212. 10.3346/jkms.2022.37.e212.
Cost-Benefit Analysis of Tafenoquine for Radical Cure of Plasmodium vivax Malaria in Korea
- Affiliations
-
- 1School of Mathematics and Computing (Computational Science and Engineering), Yonsei University, Seoul, Korea
- 2Department of Internal Medicine and AIDS Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 3Department of General Surgery, Bestian Woosong Hospital, Daejeon, Korea
- 4Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, Korea
- 5School of Mathematics and Computing, Yonsei University, Seoul, Korea
- KMID: 2531250
- DOI: http://doi.org/10.3346/jkms.2022.37.e212
Abstract
- Background
Plasmodium vivax malaria has a persistent liver stage that causes relapse, and introducing tafenoquine to suppress relapse could aid in disease eradication. Therefore, we assessed the impact of tafenoquine introduction on P. vivax malaria incidence and performed a cost-benefit analysis from the payer’s perspective.
Methods
We expanded the previously developed P. vivax malaria dynamic transmission model and calibrated it to weekly civilian malaria incidences in 2014–2018. Primaquine and tafenoquine scenarios were considered by assuming different relapse probabilities, and relapse and total P. vivax malaria cases were predicted over the next decade for each scenario. We then estimated the number of cases prevented by replacing primaquine with tafenoquine. The cost and benefit of introducing tafenoquine were obtained using medical expenditure from a nationwide database, and a cost-benefit analysis was conducted. A probabilistic sensitivity analysis was performed to assess the economic feasibility robustness of tafenoquine introduction under uncertainties of model parameters, costs, and benefits.
Results
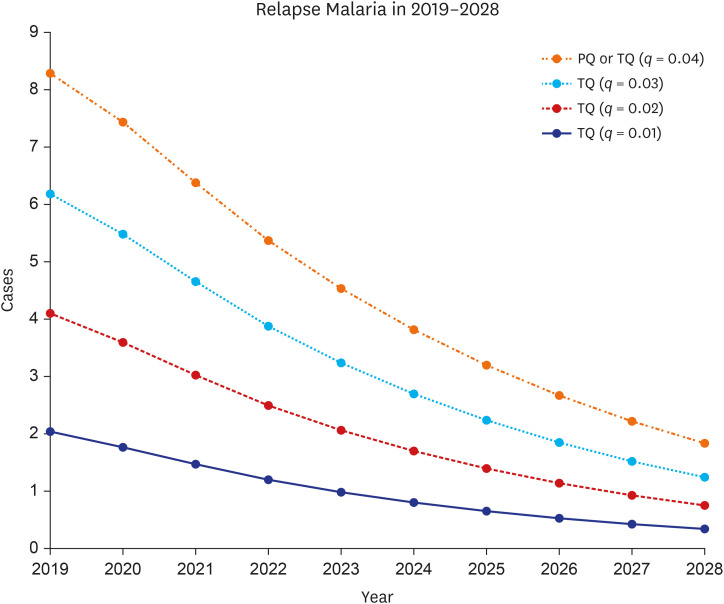
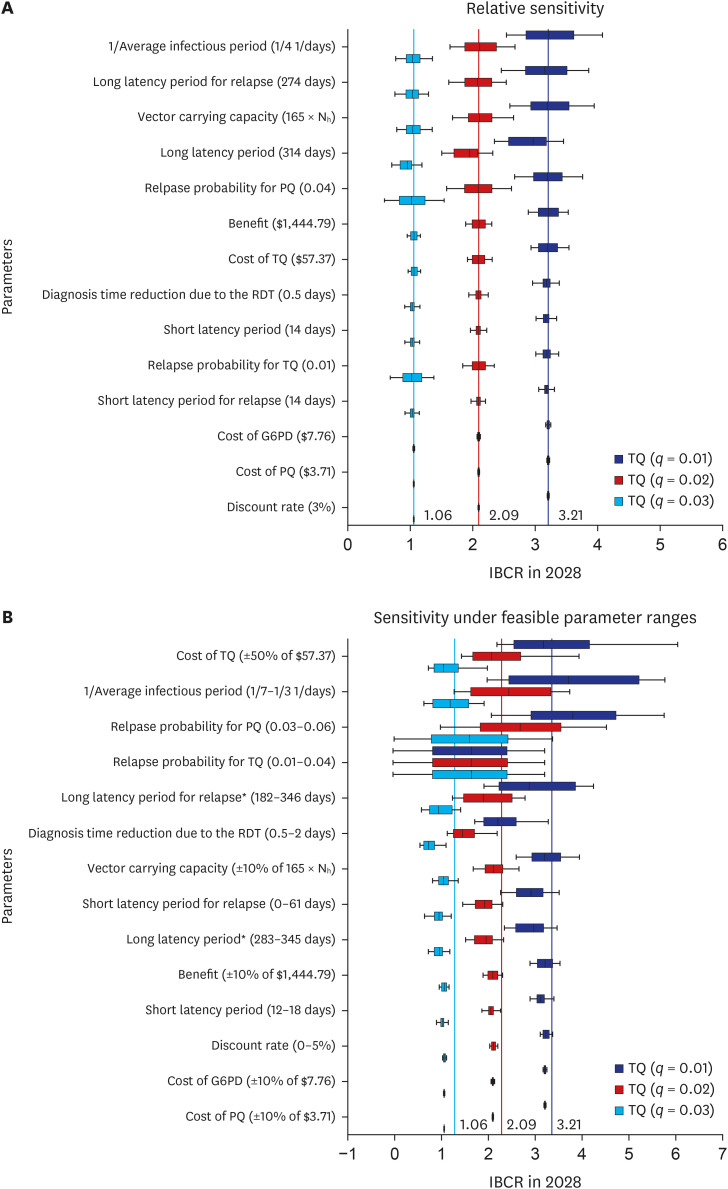
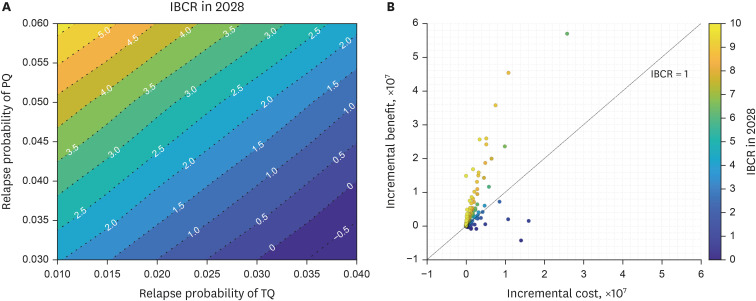
Under 0.04 primaquine relapse probability, the introduction of tafenoquine with relapse probability of 0.01 prevented 129 (12.27%) and 35 (77.78%) total and relapse cases, respectively, over the next decade. However, under the same relapse probability as primaquine, introducing tafenoquine had no additional preventative effect. The 14-day primaquine treatment cost was $3.71. The tafenoquine and the glucose-6-phosphate dehydrogenase rapid diagnostic testing cost $57.37 and $7.76, totaling $65.13. The average medical expenditure per malaria patient was estimated at $1444.79. The cost-benefit analysis results provided an incremental benefit-cost ratio (IBCR) from 0 to 3.21 as the tafenoquine relapse probability decreased from 0.04 to 0.01. The probabilistic sensitivity analysis showed an IBCR > 1, indicating that tafenoquine is beneficial, with a probability of 69.1%.
Conclusion
Tafenoquine could reduce P. vivax malaria incidence and medical costs and bring greater benefits than primaquine.
Keyword
Figure
Reference
-
1. World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges. Geneva, Switzerland: World Health Organization;2020.2. Battle KE, Lucas TC, Nguyen M, Howes RE, Nandi AK, Twohig KA, et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000-17: a spatial and temporal modelling study. Lancet. 2019; 394(10195):332–343. PMID: 31229233.
Article3. Liu Q, Jing W, Kang L, Liu J, Liu M. Trends of the global, regional and national incidence of malaria in 204 countries from 1990 to 2019 and implications for malaria prevention. J Travel Med. 2021; 28(5):taab046. PMID: 33763689.
Article5. Battle KE, Karhunen MS, Bhatt S, Gething PW, Howes RE, Golding N, et al. Geographical variation in Plasmodium vivax relapse. Malar J. 2014; 13(1):144. PMID: 24731298.
Article6. World Health Organization. Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization;2015.7. Galappaththy GN, Tharyan P, Kirubakaran R. Primaquine for preventing relapse in people with Plasmodium vivax malaria treated with chloroquine. Cochrane Database Syst Rev. 2013; 2013(10):CD004389.8. John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ, et al. Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malar J. 2012; 11(1):280. PMID: 22900786.
Article9. Pereira EA, Ishikawa EA, Fontes CJ. Adherence to Plasmodium vivax malaria treatment in the Brazilian Amazon Region. Malar J. 2011; 10(1):355. PMID: 22165853.10. Douglas NM, Poespoprodjo JR, Patriani D, Malloy MJ, Kenangalem E, Sugiarto P, et al. Unsupervised primaquine for the treatment of Plasmodium vivax malaria relapses in southern Papua: a hospital-based cohort study. PLoS Med. 2017; 14(8):e1002379. PMID: 28850568.
Article11. Taylor WR, Thriemer K, von Seidlein L, Yuentrakul P, Assawariyathipat T, Assefa A, et al. Short-course primaquine for the radical cure of Plasmodium vivax malaria: a multicentre, randomised, placebo-controlled non-inferiority trial. Lancet. 2019; 394(10202):929–938. PMID: 31327563.12. Milligan R, Daher A, Villanueva G, Bergman H, Graves PM. Primaquine alternative dosing schedules for preventing malaria relapse in people with Plasmodium vivax . Cochrane Database Syst Rev. 2020; 8:CD012656. PMID: 32816320.13. Lacerda MV, Llanos-Cuentas A, Krudsood S, Lon C, Saunders DL, Mohammed R, et al. Single-dose tafenoquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med. 2019; 380(3):215–228. PMID: 30650322.
Article14. Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014; 383(9922):1049–1058. PMID: 24360369.
Article15. Llanos-Cuentas A, Lacerda MV, Hien TT, Vélez ID, Namaik-Larp C, Chu CS, et al. Tafenoquine versus primaquine to prevent relapse of Plasmodium vivax malaria. N Engl J Med. 2019; 380(3):229–241. PMID: 30650326.
Article16. Rajapakse S, Rodrigo C, Fernando SD. Tafenoquine for preventing relapse in people with Plasmodium vivax malaria. Cochrane Database Syst Rev. 2015; 2015(4):CD010458.17. Islam N, Wright S, Lau CL, Doi SA, Mills DJ, Clark J, et al. Efficacy of a 3-day pretravel schedule of tafenoquine for malaria chemoprophylaxis: a network meta-analysis. J Travel Med. 2021; 28(5):taab057. PMID: 33834208.
Article18. Baird JK. Single loading-dose tafenoquine for malaria chemoprophylaxis during brief travel? J Travel Med. 2021; 28(5):taab081. PMID: 34050367.
Article19. Freedman DO. Tafenoquine: integrating a new drug for malaria prophylaxis into travel medicine practice. J Travel Med. 2019; 26(4):tay140. PMID: 30496585.
Article20. Ahmad SS, Rahi M, Sharma A. Relapses of Plasmodium vivax malaria threaten disease elimination: time to deploy tafenoquine in India? BMJ Glob Health. 2021; 6(2):e004558.21. Chu CS, Freedman DO. Tafenoquine and G6PD: a primer for clinicians. J Travel Med. 2019; 26(4):taz023. PMID: 30941413.
Article22. Kim S, Byun JH, Park A, Jung IH. A mathematical model for assessing the effectiveness of controlling relapse in Plasmodium vivax malaria endemic in the Republic of Korea. PLoS One. 2020; 15(1):e0227919. PMID: 31978085.23. Nekkab N, Lana R, Lacerda M, Obadia T, Siqueira A, Monteiro W, et al. Estimated impact of tafenoquine for Plasmodium vivax control and elimination in Brazil: a modelling study. PLoS Med. 2021; 18(4):e1003535. PMID: 33891582.24. Kostić M, Milosavljević MN, Stefanović S, Ranković G, Janković SM. Cost-utility of tafenoquine vs. primaquine for the radical cure (prevention of relapse) of Plasmodium vivax malaria. J Chemother. 2020; 32(1):21–29. PMID: 31524099.
Article25. Devine A, Howes RE, Price DJ, Moore KA, Ley B, Simpson JA, et al. Cost-effectiveness analysis of sex-stratified Plasmodium vivax treatment strategies using available G6PD diagnostics to accelerate access to radical cure. Am J Trop Med Hyg. 2020; 103(1):394–403. PMID: 32372747.
Article26. Kim JH, Suh J, Lee WJ, Choi H, Kim JD, Kim C, et al. Modelling the impact of rapid diagnostic tests on Plasmodium vivax malaria in South Korea: a cost-benefit analysis. BMJ Glob Health. 2021; 6(2):e004292.27. Kwak YG, Lee HK, Kim M, Um TH, Cho CR. Clinical characteristics of vivax malaria and analysis of recurred patients. Infect Chemother. 2013; 45(1):69–75. PMID: 24265952.
Article28. Korea Centers for Disease Control and Prevention. Malaria Management Guidelines 2019. Cheongju, Korea: Korea Centers for Disease Control and Prevention;2019.29. World Health Organization. WHO Guideline for Malaria, 13 July 2021. Geneva, Switzerland: World Health Organization;2021.30. Kim L, Kim JA, Kim S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol Health. 2014; 36:e2014008. PMID: 25078381.
Article31. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the national health insurance service–national sample cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46(2):e15. PMID: 26822938.
Article32. Choi MJ, Song JY, Noh JY, Yoon JG, Lee SN, Heo JY, et al. Disease burden of hospitalized community-acquired pneumonia in South Korea: analysis based on age and underlying medical conditions. Medicine (Baltimore). 2017; 96(44):e8429. PMID: 29095281.33. Korea Centers for Disease Control and Prevention. Malaria Management Guidelines 2018. Cheongju, Korea: Korea Centers for Disease Control and Prevention;2018.34. Goo YK, Ji SY, Shin HI, Moon JH, Cho SH, Lee WJ, et al. First evaluation of glucose-6-phosphate dehydrogenase (G6PD) deficiency in vivax malaria endemic regions in the Republic of Korea. PLoS One. 2014; 9(5):e97390. PMID: 24853873.
Article35. Khantikul N, Butraporn P, Kim HS, Leemingsawat S, Tempongko MA, Suwonkerd W. Adherence to antimalarial drug therapy among vivax malaria patients in northern Thailand. J Health Popul Nutr. 2009; 27(1):4–13. PMID: 19248643.
Article36. Pybus BS, Sousa JC, Jin X, Ferguson JA, Christian RE, Barnhart R, et al. CYP450 phenotyping and accurate mass identification of metabolites of the 8-aminoquinoline, anti-malarial drug primaquine. Malar J. 2012; 11(1):259. PMID: 22856549.
Article37. Pybus BS, Marcsisin SR, Jin X, Deye G, Sousa JC, Li Q, et al. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J. 2013; 12(1):212. PMID: 23782898.
Article38. Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, et al. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013; 369(14):1381–1382. PMID: 24088113.
Article39. Choi S, Choi H, Park SY, Kwak YG, Song JE, Shin SY, et al. Four times of relapse of Plasmodium vivax malaria despite primaquine treatment in a patient with impaired cytochrome P450 2D6 function. Korean J Parasitol. 2022; 60(1):39–43. PMID: 35247953.
Article40. St Jean PL, Xue Z, Carter N, Koh GC, Duparc S, Taylor M, et al. Tafenoquine treatment of Plasmodium vivax malaria: suggestive evidence that CYP2D6 reduced metabolism is not associated with relapse in the phase 2b DETECTIVE trial. Malar J. 2016; 15(1):97. PMID: 26888075.41. Chen V, Daily JP. Tafenoquine: the new kid on the block. Curr Opin Infect Dis. 2019; 32(5):407–412. PMID: 31305490.42. Lewis J, Gregorian T, Portillo I, Goad J. Drug interactions with antimalarial medications in older travelers: a clinical guide. J Travel Med. 2020; 27(1):taz089. PMID: 31776555.
Article43. Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis. 2009; 42(3):267–278. PMID: 19233695.
Article44. Howes RE, Piel FB, Patil AP, Nyangiri OA, Gething PW, Dewi M, et al. G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med. 2012; 9(11):e1001339. PMID: 23152723.
Article45. Lee W, Lee SE, Lee MJ, Noh KT. Investigation of glucose-6-phosphate dehydrogenase (G6PD) deficiency prevalence in a Plasmodium vivax-endemic area in the Republic of Korea (ROK). Malar J. 2020; 19(1):317. PMID: 32873296.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple Cerebral Infarcts Following Acute Plasmodium vivax Infection
- A Case of Plasmodium vivax Malaria Associated with Autoimmune Hemolytic Anemia
- A case of Plasmodium vivax malaria occurring during a school excursion to Pocheon-gun
- A Case of Plasmodium Vivax Malaria with Cerebral Complications
- A Case of Congenital Malaria due to Plasmodium vivax