Anat Cell Biol.
2022 Jun;55(2):217-228. 10.5115/acb.22.006.
Therapeutic effect of the mesenchymal stem cells on vigabatrin-induced retinopathy in adult male albino rat
- Affiliations
-
- 1Department of Human Anatomy & Embryology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
- 2Department of Medical Biochemistry, Faculty of Medicine, Zagazig University, Zagazig, Egypt
- KMID: 2531208
- DOI: http://doi.org/10.5115/acb.22.006
Abstract
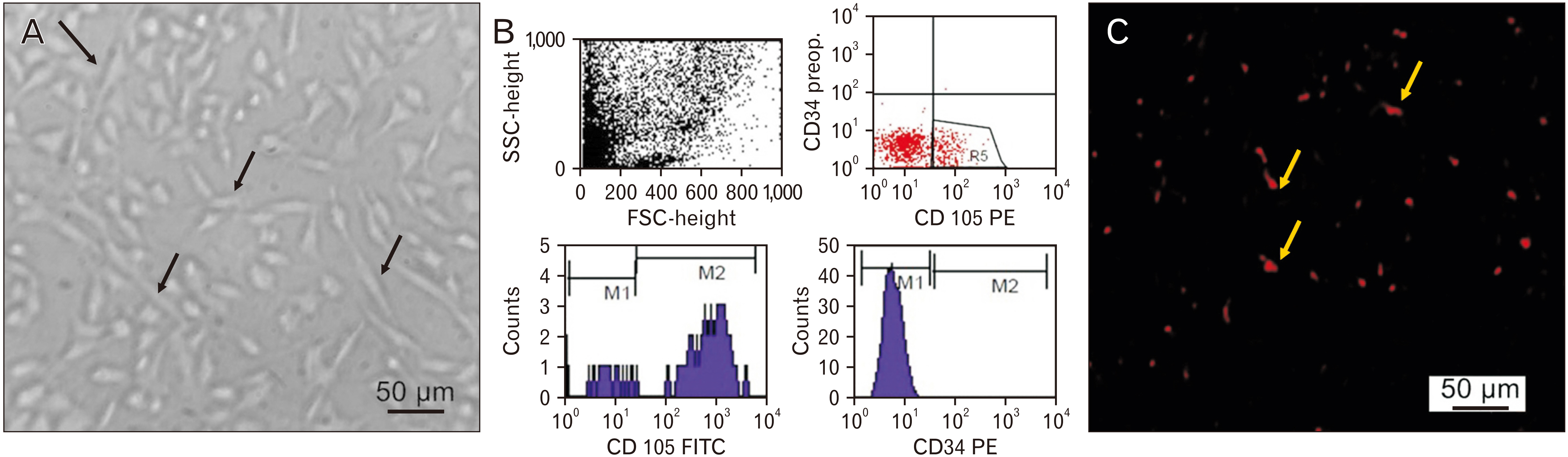
- Vigabatrin (VGB) is an effective antiepileptic drug used mainly to treat infantile spasms and refractory complex partial seizures. However, using VGB was restricted as it was known to cause retinal toxicity that appears as a severe peripheral visual field defect. Accordingly, this study was conducted to examine the histopathological and biochemical effects of VGB on the retina in adult male albino rats and assess the possible therapeutic role of mesenchymal stem cells (MSCs) against this potential toxicity. The rats were divided into three groups (control group, VGB group, and VGB/MSCs group), one week after 65 days of VGB treatment ±MSCs. The right eyeballs were prepared for histological and immunohistochemical examination, whereas the left eyeballs were prepared for real-time polymerase chain reaction analysis. Our results demonstrated that MSCs ameliorated retinal pathological changes and downregulated the expression of glial fibrillary acidic protein, vascular endothelial growth factor, and synaptophysin after VGB administration suggesting MSCs function and vascular modulating effect. Moreover, MSCs regulate retinal tissue gene expression of BAX, Bcl-2, BDNF, NGF, synapsin, interleukin (IL)-6, IL-1β, and occludin suggesting MSCs antiapoptotic and immunomodulating effect. In conclusion, MSCs administration could be a suitable therapeutic line to ameliorate VGB-induced retinopathy.
Figure
Reference
-
References
1. Holan V, Palacka K, Hermankova B. 2021; Mesenchymal stem cell-based therapy for retinal degenerative diseases: experimental models and clinical trials. Cells. 10:588. DOI: 10.3390/cells10030588. PMID: 33799995. PMCID: PMC8001847. PMID: 256a2fc6440a43bea91436dc33820daa.
Article2. Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, Costagliola C. 2015; Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res. 2015:582060. DOI: 10.1155/2015/582060. PMID: 26137497. PMCID: PMC4475523.
Article3. Shaw PX, Stiles T, Douglas C, Ho D, Fan W, Du H, Xiao X. 2016; Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol Sci. 3:196–221. DOI: 10.3934/molsci.2016.2.196. PMID: 27239555. PMCID: PMC4882104.
Article4. van Norren D, Vos JJ. 2016; Light damage to the retina: an historical approach. Eye (Lond). 30:169–72. DOI: 10.1038/eye.2015.218. PMID: 26541088. PMCID: PMC4763118.
Article5. Singh D, Jethani SL, Dubey A. 2013; Vigabatrin induced cell loss in the cerebellar cortex of albino rats. J Clin Diagn Res. 7:2555–8. DOI: 10.7860/JCDR/2013/6187.3610. PMID: 24392399. PMCID: PMC3879838. PMID: 1b1e60c86c7a4477920af91aa455cda7.
Article6. Barrett D, Yang J, Sujirakul T, Tsang SH. 2014; Vigabatrin retinal toxicity first detected with electroretinographic changes: a case report. J Clin Exp Ophthalmol. 5:1000363. DOI: 10.4172/2155-9570.1000363. PMID: 26295007. PMCID: PMC4540358.
Article7. Ben-Menachem E. 2011; Mechanism of action of vigabatrin: correcting misperceptions. Acta Neurol Scand Suppl. (192):5–15. DOI: 10.1111/j.1600-0404.2011.01596.x. PMID: 22061176.
Article8. Chan K, Hoon M, Pattnaik BR, Ver Hoeve JN, Wahlgren B, Gloe S, Williams J, Wetherbee B, Kiland JA, Vogel KR, Jansen E, Salomons G, Walters D, Roullet JB, Gibson KM, McLellan GJ. 2020; Vigabatrin-induced retinal functional alterations and second-order neuron plasticity in C57BL/6J mice. Invest Ophthalmol Vis Sci. 61:17. DOI: 10.1167/iovs.61.2.17. PMID: 32053727. PMCID: PMC7326505.
Article9. Yang J, Naumann MC, Tsai YT, Tosi J, Erol D, Lin CS, Davis RJ, Tsang SH. 2012; Vigabatrin-induced retinal toxicity is partially mediated by signaling in rod and cone photoreceptors. PLoS One. 7:e43889. DOI: 10.1371/journal.pone.0043889. PMID: 22970106. PMCID: PMC3431405.
Article10. Ravindran J, Blumbergs P, Crompton J, Pietris G, Waddy H. 2001; Visual field loss associated with vigabatrin: pathological correlations. J Neurol Neurosurg Psychiatry. 70:787–9. DOI: 10.1136/jnnp.70.6.787. PMID: 11385015. PMCID: PMC1737373.
Article11. Hawker MJ, Astbury NJ. 2008; The ocular side effects of vigabatrin (Sabril): information and guidance for screening. Eye (Lond). 22:1097–8. DOI: 10.1038/eye.2008.139. PMID: 18497834.
Article12. Waterhouse EJ, Mims KN, Gowda SN. 2009; Treatment of refractory complex partial seizures: role of vigabatrin. Neuropsychiatr Dis Treat. 5:505–15. DOI: 10.2147/NDT.S5236. PMID: 19851518. PMCID: PMC2762367.
Article13. Riikonen R, Rener-Primec Z, Carmant L, Dorofeeva M, Hollody K, Szabo I, Krajnc BS, Wohlrab G, Sorri I. 2015; Does vigabatrin treatment for infantile spasms cause visual field defects? An international multicentre study. Dev Med Child Neurol. 57:60–7. DOI: 10.1111/dmcn.12573. PMID: 25145415.
Article14. Durbin S, Mirabella G, Buncic JR, Westall CA. 2009; Reduced grating acuity associated with retinal toxicity in children with infantile spasms on vigabatrin therapy. Invest Ophthalmol Vis Sci. 50:4011–6. DOI: 10.1167/iovs.08-3237. PMID: 19279311. PMCID: PMC3880356.
Article15. Hou HY, Liang HL, Wang YS, Zhang ZX, Wang BR, Shi YY, Dong X, Cai Y. 2010; A therapeutic strategy for choroidal neovascularization based on recruitment of mesenchymal stem cells to the sites of lesions. Mol Ther. 18:1837–45. DOI: 10.1038/mt.2010.144. PMID: 20647999. PMCID: PMC2951561.
Article16. Achyut BR, Varma NR, Arbab AS. 2014; Application of umbilical cord blood derived stem cells in diseases of the nervous system. J Stem Cell Res Ther. 4:1000202. DOI: 10.4172/2157-7633.1000202. PMID: 25599002. PMCID: PMC4296316.
Article17. Chen T, Wang F, Wu M, Wang ZZ. 2015; Development of hematopoietic stem and progenitor cells from human pluripotent stem cells. J Cell Biochem. 116:1179–89. DOI: 10.1002/jcb.25097. PMID: 25740540. PMCID: PMC4452944.
Article18. Nancarrow-Lei R, Mafi P, Mafi R, Khan W. 2017; A systemic review of adult mesenchymal stem cell sources and their multilineage differentiation potential relevant to musculoskeletal tissue repair and regeneration. Curr Stem Cell Res Ther. 12:601–10. DOI: 10.2174/1574888X12666170608124303. PMID: 28595566.
Article19. Ding SLS, Kumar S, Mok PL. 2017; Cellular reparative mechanisms of mesenchymal stem cells for retinal diseases. Int J Mol Sci. 18:1406. DOI: 10.3390/ijms18081406. PMID: 28788088. PMCID: PMC5577990.
Article20. Jammoul F, Wang Q, Nabbout R, Coriat C, Duboc A, Simonutti M, Dubus E, Craft CM, Ye W, Collins SD, Dulac O, Chiron C, Sahel JA, Picaud S. 2009; Taurine deficiency is a cause of vigabatrin-induced retinal phototoxicity. Ann Neurol. 65:98–107. DOI: 10.1002/ana.21526. PMID: 19194884. PMCID: PMC2665303.
Article21. Laitinen A, Laine J. 2007; Isolation of mesenchymal stem cells from human cord blood. Curr Protoc Stem Cell Biol. Chapter 2:Unit 2A.3. DOI: 10.1002/9780470151808.sc02a03s1. PMID: 18785175.
Article22. Shehata AS, Mohamed DA, Hagras SM, El-Beah SM, Elnegris HM. 2021; The role of hesperidin in ameliorating retinal changes in rats with experimentally induced type 1 diabetes mellitus and the active role of vascular endothelial growth factor and glial fibrillary acidic protein. Anat Cell Biol. 54:465–78. DOI: 10.5115/acb.21.105. PMID: 34936987. PMCID: PMC8693142.
Article23. Suvarna SK, Layton C, Bancroft JD. 2013. Bancroft's theory and practice of histological techniques. 7th ed. Churchill Livingstone Elsevier;Edinburgh: DOI: 10.1002/9780470151808.sc02a03s1.24. Chen H, Weber AJ. 2002; Expression of glial fibrillary acidic protein and glutamine synthetase by Müller cells after optic nerve damage and intravitreal application of brain-derived neurotrophic factor. Glia. 38:115–25. DOI: 10.1002/glia.10061. PMID: 11948805.
Article25. Dan C, Jian-Bin T, Hui W, Le-Ping Z, Jin Z, Ju-Fang H, Xue-Gang L. 2008; Synaptophysin expression in rat retina following acute high intraocular pressure. Acta Histochem Cytochem. 41:173–8. DOI: 10.1267/ahc.08034. PMID: 19180202. PMCID: PMC2629489.
Article26. Youssef NS, Said AM. 2014; Immunohistochemical expression of CD117 and vascular endothelial growth factor in retinoblastoma: possible targets of new therapies. Int J Clin Exp Pathol. 7:5725–37. PMID: 25337214. PMCID: PMC4203185.27. El-Gohari K, Bahei-Eldin I, Habib E, Saad S, Rady H, Said A. 2016; Neuroprotection of the rat's retinal ganglion cells against glutamate-induced toxicity. J Egypt Ophthalmol Soc. 109:135–44. DOI: 10.4103/2090-0686.202261.
Article28. Dagar S, Nagar S, Goel M, Cherukuri P, Dhingra NK. 2014; Loss of photoreceptors results in upregulation of synaptic proteins in bipolar cells and amacrine cells. PLoS One. 9:e90250. DOI: 10.1371/journal.pone.0090250. PMID: 24595229. PMCID: PMC3942420. PMID: c6dd59d9698044efb995864809f96b45.
Article29. Liu X, Wang D, Liu Y, Luo Y, Ma W, Xiao W, Yu Q. 2010; Neuronal-driven angiogenesis: role of NGF in retinal neovascularization in an oxygen-induced retinopathy model. Invest Ophthalmol Vis Sci. 51:3749–57. DOI: 10.1167/iovs.09-4226. PMID: 20207957.
Article30. Tao Y, Ding L, Yao A, Yang Z, Yang Q, Qin L, Yu L, Gao Y, Huang YF, Li Z, Teng D. 2018; Intravitreous delivery of Αb-crystallin ameliorates N-methyl-N-nitrosourea induced photoreceptor degeneration in mice: an in vivo and ex vivo study. Cell Physiol Biochem. 48:2147–60. DOI: 10.1159/000492557. PMID: 30110696.
Article31. Vogel KR, Pearl PL, Theodore WH, McCarter RC, Jakobs C, Gibson KM. 2013; Thirty years beyond discovery--clinical trials in succinic semialdehyde dehydrogenase deficiency, a disorder of GABA metabolism. J Inherit Metab Dis. 36:401–10. DOI: 10.1007/s10545-012-9499-5. PMID: 22739941. PMCID: PMC4349389.
Article32. Duboc A, Hanoteau N, Simonutti M, Rudolf G, Nehlig A, Sahel JA, Picaud S. 2004; Vigabatrin, the GABA-transaminase inhibitor, damages cone photoreceptors in rats. Ann Neurol. 55:695–705. DOI: 10.1002/ana.20081. PMID: 15122710.
Article33. Jammoul F, Dégardin J, Pain D, Gondouin P, Simonutti M, Dubus E, Caplette R, Fouquet S, Craft CM, Sahel JA, Picaud S. 2010; Taurine deficiency damages photoreceptors and retinal ganglion cells in vigabatrin-treated neonatal rats. Mol Cell Neurosci. 43:414–21. DOI: 10.1016/j.mcn.2010.01.008. PMID: 20132888. PMCID: PMC2864319.
Article34. Wang QP, Jammoul F, Duboc A, Gong J, Simonutti M, Dubus E, Craft CM, Ye W, Sahel JA, Picaud S. 2008; Treatment of epilepsy: the GABA-transaminase inhibitor, vigabatrin, induces neuronal plasticity in the mouse retina. Eur J Neurosci. 27:2177–87. DOI: 10.1111/j.1460-9568.2008.06175.x. PMID: 18412635. PMCID: PMC2933832.
Article35. Gaucher D, Arnault E, Husson Z, Froger N, Dubus E, Gondouin P, Dherbécourt D, Degardin J, Simonutti M, Fouquet S, Benahmed MA, Elbayed K, Namer IJ, Massin P, Sahel JA, Picaud S. 2012; Taurine deficiency damages retinal neurones: cone photoreceptors and retinal ganglion cells. Amino Acids. 43:1979–93. DOI: 10.1007/s00726-012-1273-3. PMID: 22476345. PMCID: PMC3472058.
Article36. Zhou L, Wang H, Luo J, Xiong K, Zeng L, Chen D, Huang J. 2014; Regulatory effects of inhibiting the activation of glial cells on retinal synaptic plasticity. Neural Regen Res. 9:385–93. DOI: 10.4103/1673-5374.128240. PMID: 25206825. PMCID: PMC4146193.
Article37. Ponjavic V, Andréasson S. 2001; Multifocal ERG and full-field ERG in patients on long-term vigabatrin medication. Doc Ophthalmol. 102:63–72. DOI: 10.1023/A:1017589301855. PMID: 11475366.38. Lewis GP, Fisher SK. 2003; Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 230:263–90. DOI: 10.1016/S0074-7696(03)30005-1. PMID: 14692684.
Article39. Sethi CS, Lewis GP, Fisher SK, Leitner WP, Mann DL, Luthert PJ, Charteris DG. 2005; Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 46:329–42. DOI: 10.1167/iovs.03-0518. PMID: 15623793.
Article40. Xue LP, Lu J, Cao Q, Hu S, Ding P, Ling EA. 2006; Müller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience. 139:723–32. DOI: 10.1016/j.neuroscience.2005.12.032. PMID: 16458441.
Article41. Dijk F, Bergen AA, Kamphuis W. 2007; GAP-43 expression is upregulated in retinal ganglion cells after ischemia/reperfusion-induced damage. Exp Eye Res. 84:858–67. DOI: 10.1016/j.exer.2007.01.006. PMID: 17343850.
Article42. Ferrara N. 1999; Molecular and biological properties of vascular endothelial growth factor. J Mol Med (Berl). 77:527–43. DOI: 10.1007/s001099900019. PMID: 10494799.
Article43. McKay JS, Steele SJ, Ahmed G, Johnson E, Ratcliffe K. 2009; An antibody panel for immunohistochemical analysis of the retina in Davidson's-fixed, paraffin-embedded eyes of rats. Exp Toxicol Pathol. 61:91–100. DOI: 10.1016/j.etp.2008.06.005. PMID: 18718747.
Article44. Marchena M, Lara J, Aijón J, Germain F, de la Villa P, Velasco A. 2011; The retina of the PCD/PCD mouse as a model of photoreceptor degeneration. A structural and functional study. Exp Eye Res. 93:607–17. DOI: 10.1016/j.exer.2011.07.010. PMID: 21824473.
Article45. Barber AJ, Antonetti DA. 2003; Mapping the blood vessels with paracellular permeability in the retinas of diabetic rats. Invest Ophthalmol Vis Sci. 44:5410–6. DOI: 10.1167/iovs.03-0244. PMID: 14638745.
Article46. Yu C, Yang K, Meng X, Cao B, Wang F. 2020; Downregulation of long noncoding RNA MIAT in the retina of diabetic rats with tail-vein injection of human umbilical-cord mesenchymal stem cells. Int J Med Sci. 17:591–8. DOI: 10.7150/ijms.38078. PMID: 32210708. PMCID: PMC7085208.
Article47. Mao F, Wu Y, Tang X, Wang J, Pan Z, Zhang P, Zhang B, Yan Y, Zhang X, Qian H, Xu W. 2017; Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease through the regulation of 15-LOX-1 in macrophages. Biotechnol Lett. 39:929–38. DOI: 10.1007/s10529-017-2315-4. PMID: 28258529.
Article48. Bamforth SD, Lightman SL, Greenwood J. 1997; Interleukin-1 beta-induced disruption of the retinal vascular barrier of the central nervous system is mediated through leukocyte recruitment and histamine. Am J Pathol. 150:329–40. PMID: 9006348. PMCID: PMC1858506.49. Qaum T, Xu Q, Joussen AM, Clemens MW, Qin W, Miyamoto K, Hassessian H, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP. 2001; VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 42:2408–13. PMID: 11527957.50. Meng HB, Gong J, Zhou B, Hua J, Yao L, Song ZS. 2013; Therapeutic effect of human umbilical cord-derived mesenchymal stem cells in rat severe acute pancreatitis. Int J Clin Exp Pathol. 6:2703–12. PMID: 24294357. PMCID: PMC3843251.51. Li Z, Wang J, Gao F, Zhang J, Tian H, Shi X, Lian C, Sun Y, Li W, Xu JY, Li P, Zhang J, Gao Z, Xu J, Wang F, Lu L, Xu GT. 2016; Human adipose-derived stem cells delay retinal degeneration in royal college of surgeons rats through anti-apoptotic and VEGF-mediated neuroprotective effects. Curr Mol Med. 16:553–66. DOI: 10.2174/1566524016666160607090538. PMID: 27280496.
Article52. Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U. 2007; Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 245:414–22. DOI: 10.1007/s00417-006-0382-7. PMID: 16896916.
Article53. Zhang Y, Wang W. 2010; Effects of bone marrow mesenchymal stem cell transplantation on light-damaged retina. Invest Ophthalmol Vis Sci. 51:3742–8. DOI: 10.1167/iovs.08-3314. PMID: 20207980.
Article54. Labrador-Velandia S, Alonso-Alonso ML, Di Lauro S, García-Gutierrez MT, ivastava GK Sr, Pastor JC, Fernandez-Bueno I. 2019; Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp Eye Res. 185:107671. DOI: 10.1016/j.exer.2019.05.011. PMID: 31108056.
Article55. Wang S, Lu B, Girman S, Duan J, McFarland T, Zhang QS, Grompe M, Adamus G, Appukuttan B, Lund R. 2010; Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLoS One. 5:e9200. DOI: 10.1371/journal.pone.0009200. PMID: 20169166. PMCID: PMC2821411. PMID: d539180245324ab18e253cd221a049a4.
Article56. Jindal N, Mukhopadhyay A, Anand A. 2012; The emerging role of stem cells in ocular neurodegeneration: hype or hope? Mol Cell Biochem. 365:65–76. DOI: 10.1007/s11010-012-1244-8. PMID: 22290231.
Article57. Li N, Li XR, Yuan JQ. 2009; Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefes Arch Clin Exp Ophthalmol. 247:503–14. DOI: 10.1007/s00417-008-1009-y. PMID: 19084985.
Article58. Jindal N, Banik A, Prabhakar S, Vaiphie K, Anand A. 2017; Alteration of neurotrophic factors after transplantation of bone marrow derived Lin-ve stem cell in NMDA-induced mouse model of retinal degeneration. J Cell Biochem. 118:1699–711. DOI: 10.1002/jcb.25827. PMID: 27935095.
Article59. Xu W, Wang X, Xu G, Guo J. 2013; Light-induced retinal injury enhanced neurotrophins secretion and neurotrophic effect of mesenchymal stem cells in vitro. Arq Bras Oftalmol. 76:105–10. DOI: 10.1590/S0004-27492013000200010. PMID: 23828471.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Possible Role of Mesenchymal Stem Cells Therapy in the Repair of Experimentally Induced Colitis in Male Albino Rats
- Possible Therapeutic Effect of Stem Cell in Atherosclerosis in Albino Rats. A Histological and Immunohistochemical Study
- Effect of Bone Marrow Derived Mesenchymal Stem Cells on Healing of Induced Full-Thickness Skin Wounds in Albino Rat
- Experimental Study on the Effect of Intravenous Stem Cell Therapy on Intestinal Ischemia Reperfusion Induced Myocardial Injury
- Effect of Stem Cell Therapy on Adriamycin Induced Tubulointerstitial Injury