Korean J Physiol Pharmacol.
2022 Jul;26(4):255-262. 10.4196/kjpp.2022.26.4.255.
Oxytocin-induced endothelial nitric oxide dependent vasorelaxation and ERK1/2-mediated vasoconstriction in the rat aorta
- Affiliations
-
- 1Department of Physiology, Shenyang Medical University, Shenyang 110034, P.R. China
- KMID: 2530945
- DOI: http://doi.org/10.4196/kjpp.2022.26.4.255
Abstract
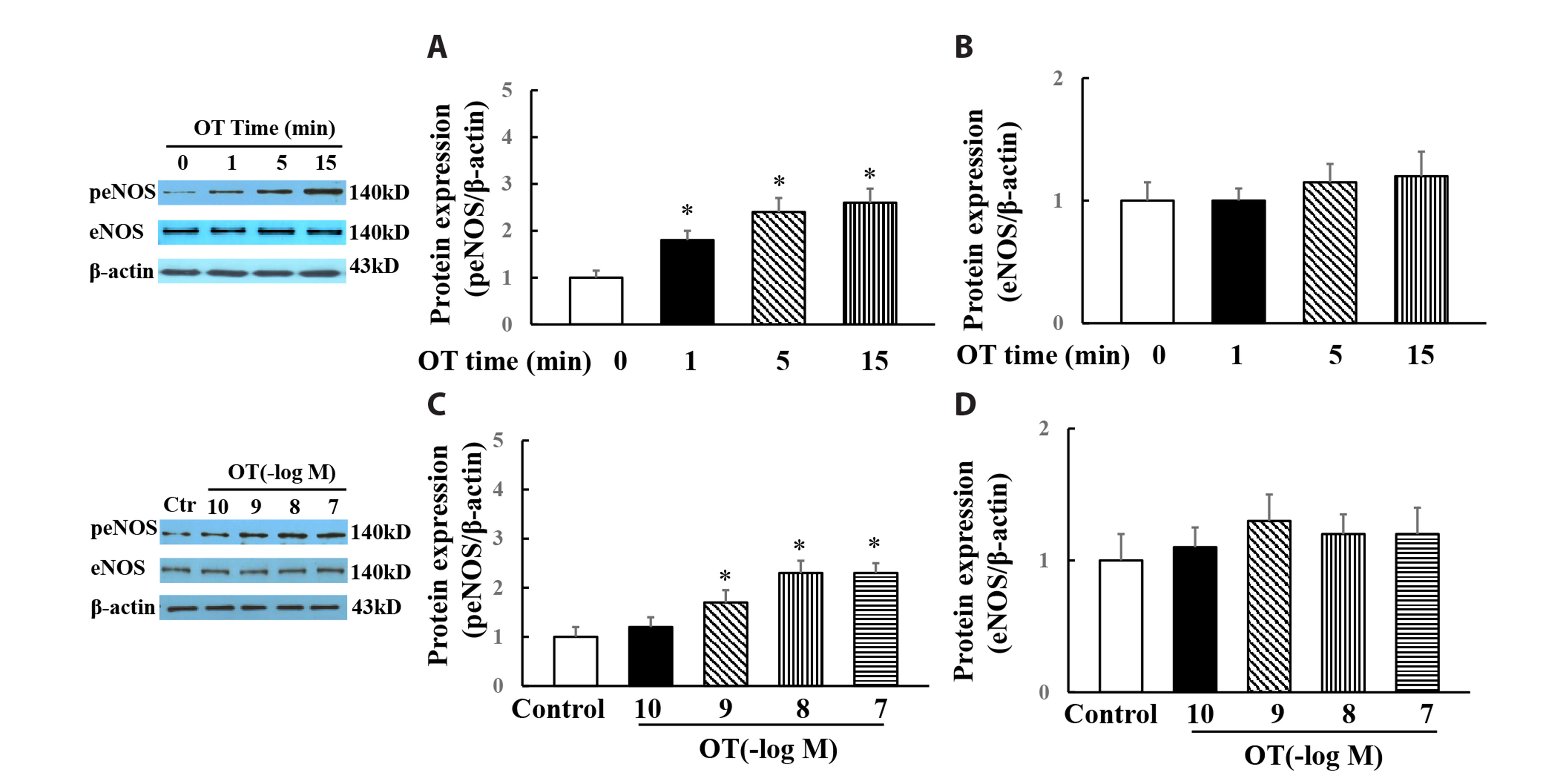
- Oxytocin is a neuropeptide produced primarily in the hypothalamus and plays an important role in the regulation of mammalian birth and lactation. It has been shown that oxytocin has important cardiovascular protective effects. Here we investigated the effects of oxytocin on vascular reactivity and underlying the mechanisms in human umbilical vein endothelial cells (HUVECs) in vitro and in rat aorta ex vivo. Oxytocin increased phospho-eNOS (Ser 1177) and phospho-Akt (Ser 473) expression in HUVECs in vitro and the aorta of rat ex vivo. Wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase (PI3K), inhibited oxytocin-induced Akt and eNOS phosphorylation. In the rat aortic rings, oxytocin induced a biphasic vascular reactivity: oxytocin at low dose (10-9–10
-8 M) initiated a vasorelaxation followed by a vasoconstriction at high dose (10-7 M). L-NAME (a nitric oxide synthase inhibitor), endothelium removal or wortmannin abolished oxytocin-induced vasorelaxation, and slightly enhanced oxytocin-induced vasoconstriction. Atosiban, an oxytocin/ vasopressin 1a receptor inhibitor, totally blocked oxytocin-induced relaxation and vasoconstriction. PD98059 (ERK1/2 inhibitor) partially inhibited oxytocin-induced vasoconstriction. Oxytocin also increased aortic phospho-ERK1/2 expression, which was reduced by either atosiban or PD98059, suggesting that oxytocin-induced vasoconstriction was partially mediated by oxytocin/V1aR activation of ERK1/2. The present study demonstrates that oxytocin can activate different signaling pathways to cause vasorelaxation or vasoconstriction. Oxytocin stimulation of PI3K/eNOS-derived nitric oxide may participate in maintenance of cardiovascular homeostasis, and different vascular reactivities to low or high dose of oxytocin suggest that oxytocin may have different regulatory effects on vascular tone under physiological or pathophysiological conditions.
Keyword
Figure
Reference
-
1. Jurek B, Neumann ID. 2018; The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 98:1805–1908. DOI: 10.1152/physrev.00031.2017. PMID: 29897293. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85048730134&origin=inward.
Article2. Jankowski M, Broderick TL, Gutkowska J. 2020; The role of oxytocin in cardiovascular protection. Front Psychol. 11:2139. DOI: 10.3389/fpsyg.2020.02139. PMID: 32982875. PMCID: PMC7477297. PMID: 9bb5c602a4d44bc881e1fbc70afc72d5. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85090777237&origin=inward.
Article3. Szczepanska-Sadowska E, Wsol A, Cudnoch-Jedrzejewska A, Żera T. 2021; Complementary role of oxytocin and vasopressin in cardiovascular regulation. Int J Mol Sci. 22:11465. DOI: 10.3390/ijms222111465. PMID: 34768894. PMCID: PMC8584236. PMID: ca08fa6e6f4040b1aabddb4b719c4eb9. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85117595253&origin=inward.
Article4. McKay EC, Counts SE. 2020; Oxytocin receptor signaling in vascular function and stroke. Front Neurosci. 14:574499. DOI: 10.3389/fnins.2020.574499. PMID: 33071746. PMCID: PMC7544744. PMID: e3b8178eaa9d4cd08db8550fff61dfed. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85092278060&origin=inward.
Article5. Liu S, Pan S, Tan J, Zhao W, Liu F. 2017; Oxytocin inhibits ox-LDL-induced adhesion of monocytic THP-1 cells to human brain microvascular endothelial cells. Toxicol Appl Pharmacol. 337:104–110. DOI: 10.1016/j.taap.2017.10.022. PMID: 29104011. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85033378308&origin=inward.
Article6. Nation DA, Szeto A, Mendez AJ, Brooks LG, Zaias J, Herderick EE, Gonzales J, Noller CM, Schneiderman N, McCabe PM. 2010; Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated ApoE-/- mice. Psychosom Med. 72:376–382. DOI: 10.1097/PSY.0b013e3181d74c48. PMID: 20368478. PMCID: PMC4784697. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77952425954&origin=inward.
Article7. Japundžić-Žigon N, Lozić M, Šarenac O, Murphy D. 2020; Vasopressin & oxytocin in control of the cardiovascular system: an updated review. Curr Neuropharmacol. 18:14–33. DOI: 10.2174/1570159X17666190717150501. PMID: 31544693. PMCID: PMC7327933. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077294829&origin=inward.
Article8. Petersson M, Lundeberg T, Uvnäs-Moberg K. 1997; Oxytocin decreases blood pressure in male but not in female spontaneously hypertensive rats. J Auton Nerv Syst. 66:15–18. DOI: 10.1016/S0165-1838(97)00040-4. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0031563516&origin=inward.
Article9. Gutkowska J, Aliou Y, Lavoie JL, Gaab K, Jankowski M, Broderick TL. 2016; Oxytocin decreases diurnal and nocturnal arterial blood pressure in the conscious unrestrained spontaneously hypertensive rat. Pathophysiology. 23:111–121. DOI: 10.1016/j.pathophys.2016.03.003. PMID: 27020751. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84962184528&origin=inward.
Article10. Somjit M, Surojananon J, Kongwattanakul K, Kasemsiri C, Sirisom M, Prawannoa K, Thepsuthammarat K, Komwilaisak R. 2020; Comparison of low dose versus high dose of oxytocin for initiating uterine contraction during cesarean delivery: a randomized, controlled, non-inferiority trial. Int J Womens Health. 12:667–673. DOI: 10.2147/IJWH.S260073. PMID: 32904472. PMCID: PMC7455765. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85090506827&origin=inward.11. Wsol A, Wojno O, Puchalska L, Wrzesien R, Szczepanska-Sadowska E, Cudnoch-Jedrzejewska A. 2020; Impaired hypotensive effects of centrally acting oxytocin in SHR and WKY rats exposed to chronic mild stress. Am J Physiol Regul Integr Comp Physiol. 318:R160–R172. DOI: 10.1152/ajpregu.00050.2019. PMID: 31644319. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077762246&origin=inward.
Article12. Oyama H, Suzuki Y, Satoh S, Kajita Y, Takayasu M, Shibuya M, Sugita K. 1993; Role of nitric oxide in the cerebral vasodilatory responses to vasopressin and oxytocin in dogs. J Cereb Blood Flow Metab. 13:285–290. DOI: 10.1038/jcbfm.1993.35. PMID: 8436620. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0027476776&origin=inward.
Article13. Suzuki Y, Satoh S, Kimura M, Oyama H, Asano T, Shibuya M, Sugita K. 1992; Effects of vasopressin and oxytocin on canine cerebral circulation in vivo. J Neurosurg. 77:424–431. DOI: 10.3171/jns.1992.77.3.0424. PMID: 1506890. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0026641189&origin=inward.
Article14. Katusic ZS, Shepherd JT, Vanhoutte PM. 1986; Oxytocin causes endothelium-dependent relaxations of canine basilar arteries by activating V1-vasopressinergic receptors. J Pharmacol Exp Ther. 236:166–170. PMID: 3001282. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0022645675&origin=inward.15. Cai R, Hao Y, Liu YY, Huang L, Yao Y, Zhou MS. 2020; Tumor necrosis factor alpha deficiency improves endothelial function and cardiovascular injury in deoxycorticosterone acetate/salt-hypertensive mice. Biomed Res Int. 2020:3921074. DOI: 10.1155/2020/3921074. PMID: 32190663. PMCID: PMC7064859. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082006872&origin=inward.
Article16. Reiss AB, Glass DS, Lam E, Glass AD, De Leon J, Kasselman LJ. 2019; Oxytocin: potential to mitigate cardiovascular risk. Peptides. 117:170089. DOI: 10.1016/j.peptides.2019.05.001. PMID: 31112739. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85067972878&origin=inward.
Article17. Faghihi M, Alizadeh AM, Khori V, Latifpour M, Khodayari S. 2012; The role of nitric oxide, reactive oxygen species, and protein kinase C in oxytocin-induced cardioprotection in ischemic rat heart. Peptides. 37:314–319. DOI: 10.1016/j.peptides.2012.08.001. PMID: 22902709. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84865337214&origin=inward.
Article18. Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. 2008; Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab. 295:E1495–E1501. DOI: 10.1152/ajpendo.90718.2008. PMID: 18940936. PMCID: PMC2603556. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=57349088269&origin=inward.
Article19. Hussien NI, Mousa AM. 2016; Could nitric oxide be a mediator of action of oxytocin on myocardial injury in rats? (biochemical, histological and immunohistochemical study). Gen Physiol Biophys. 35:353–362. DOI: 10.4149/gpb_2015049. PMID: 27226256. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84974628199&origin=inward.
Article20. Szeto A, Rossetti MA, Mendez AJ, Noller CM, Herderick EE, Gonzales JA, Schneiderman N, McCabe PM. 2013; Oxytocin administration attenuates atherosclerosis and inflammation in Watanabe Heritable Hyperlipidemic rabbits. Psychoneuroendocrinology. 38:685–693. DOI: 10.1016/j.psyneuen.2012.08.009. PMID: 22998949. PMCID: PMC3543511. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84876090828&origin=inward.
Article21. Gonzalez-Reyes A, Menaouar A, Yip D, Danalache B, Plante E, Noiseux N, Gutkowska J, Jankowski M. 2015; Molecular mechanisms underlying oxytocin-induced cardiomyocyte protection from simulated ischemia-reperfusion. Mol Cell Endocrinol. 412:170–181. DOI: 10.1016/j.mce.2015.04.028. PMID: 25963797. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84939573492&origin=inward.
Article22. Anvari MA, Imani A, Faghihi M, Karimian SM, Moghimian M, Khansari M. 2012; The administration of oxytocin during early reperfusion, dose-dependently protects the isolated male rat heart against ischemia/reperfusion injury. Eur J Pharmacol. 682:137–141. DOI: 10.1016/j.ejphar.2012.02.029. PMID: 22406244. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84859110002&origin=inward.
Article23. Gutkowska J, Jankowski M, Antunes-Rodrigues J. 2014; The role of oxytocin in cardiovascular regulation. Braz J Med Biol Res. 47:206–214. DOI: 10.1590/1414-431X20133309. PMID: 24676493. PMCID: PMC3982941. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84898896689&origin=inward.
Article24. Cattaneo MG, Chini B, Vicentini LM. 2008; Oxytocin stimulates migration and invasion in human endothelial cells. Br J Pharmacol. 153:728–736. DOI: 10.1038/sj.bjp.0707609. PMID: 18059319. PMCID: PMC2259201. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=39449121993&origin=inward.
Article25. Florian M, Jankowski M, Gutkowska J. 2010; Oxytocin increases glucose uptake in neonatal rat cardiomyocytes. Endocrinology. 151:482–491. DOI: 10.1210/en.2009-0624. PMID: 20008042. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=74949108334&origin=inward.
Article26. Zhou MS, Schulman IH, Raij L. 2004; Nitric oxide, angiotensin II, and hypertension. Semin Nephrol. 24:366–378. DOI: 10.1016/j.semnephrol.2004.04.008. PMID: 15252776. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=3142540898&origin=inward.
Article27. Japundžić-Žigon N. 2013; Vasopressin and oxytocin in control of the cardiovascular system. Curr Neuropharmacol. 11:218–230. DOI: 10.2174/1570159X11311020008. PMID: 23997756. PMCID: PMC3637675. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84876703862&origin=inward.
Article28. Wiśniewski K. 2019; Design of oxytocin analogs. Methods Mol Biol. 2001:235–271. DOI: 10.1007/978-1-4939-9504-2_11. PMID: 31134574. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85066281457&origin=inward.
Article29. Zhong M, Yang M, Sanborn BM. 2003; Extracellular signal-regulated kinase 1/2 activation by myometrial oxytocin receptor involves GαqGβγ and epidermal growth factor receptor tyrosine kinase activation. Endocrinology. 144:2947–2956. DOI: 10.1210/en.2002-221039. PMID: 12810550. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0038675282&origin=inward.
Article30. Wang P, Qin D, Wang YF. 2017; Oxytocin rapidly changes astrocytic GFAP plasticity by differentially modulating the expressions of pERK 1/2 and protein kinase A. Front Mol Neurosci. 10:262. DOI: 10.3389/fnmol.2017.00262. PMID: 28860967. PMCID: PMC5559427. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85027869582&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vascular Reactivity by Purinoceptor Activation in Rat Inferior Vena Cava
- Bacterial Lipopolysaccharide - Induced Generation of Nitric Oxide is Mediated by ERK1 and JNK1 / SAPK in Primary Rat Neonatal Astrocytes
- Impaired endothelium-dependent relaxation is mediated by reduced production of nitric oxide in the streptozotocin-induced diabetic rats
- Nitric Oxide/cGMP-Independent Vasorelaxation Enhanced by L-Arginine
- Dexmedetomidine inhibits vasoconstriction via activation of endothelial nitric oxide synthase