J Korean Med Sci.
2022 Jun;37(24):e190. 10.3346/jkms.2022.37.e190.
Impact of COVID-19 on Clinicopathological Spectrum of Pityriasis Rosea in Korea
- Affiliations
-
- 1Department of Dermatology, Korea University College of Medicine, Seoul, Korea
- 2Department of Anatomy, Korea University College of Medicine, Seoul, Korea
- KMID: 2530503
- DOI: http://doi.org/10.3346/jkms.2022.37.e190
Abstract
- Background
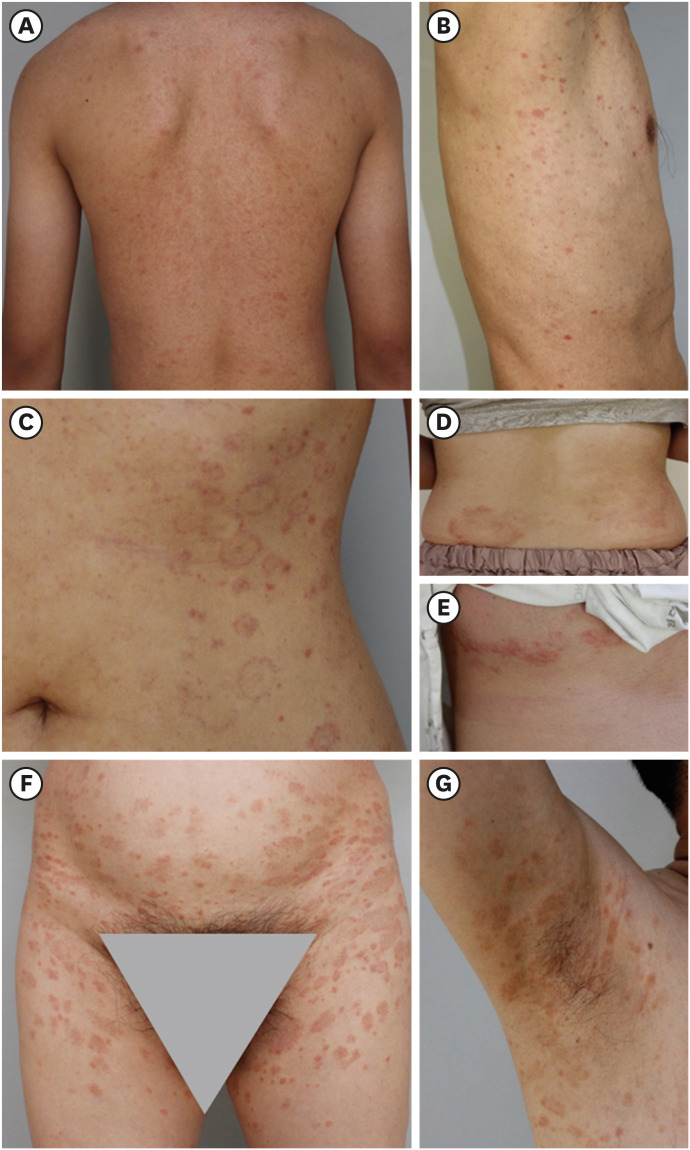
Pityriasis rosea (PR) is a papulosquamous eruption with generally unknown origin but suspected to be related to viral etiologies. The clinicopathological spectrum of several disorders with viral etiologies has been altered after the coronavirus disease 2019 (COVID-19) pandemic. The author group could experience coherent histological alterations in PR after the COVID-19 pandemic. This study aimed to investigate how the clinicopathological findings of PR were changed after the COVID-19 pandemic.
Methods
Patients (n = 11) diagnosed with PR based on the clinical manifestations and skin biopsies between February 2018 and October 2019 and 11 patients in February 2020 and October 2021 were retrospectively analyzed by investigating the medical records.
Results
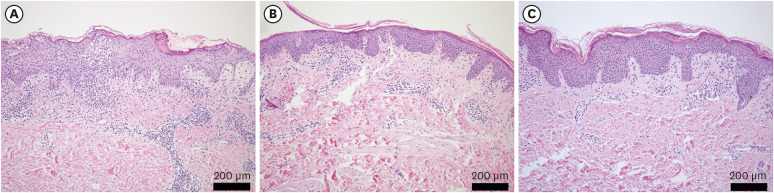
The patients with PR during the COVID-19 pandemic demonstrated statistically significant histopathological alterations from classic brisk and dense infiltration pattern to dormant and sparse infiltration and psoriasiform-dominant patterns (P = 0.019). PR was associated with more frequent pruritus during the pandemic period (P = 0.027).
Conclusion
In conclusion, PR demonstrated a significant histopathological alteration with more frequent pruritus during the COVID-19 pandemic. The comparative results about clinicopathological findings of PR will provide a useful reference for dermatologists in the diagnostic process of PR in the COVID-19 pandemic.
Keyword
Figure
Reference
-
1. Clark M, Gudjonsson JE. Pityriasis rosea. Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, editors. Fitzpatrick’s Dermatology. 9th ed. New York, NY, USA: McGraw-Hill Education;2019. p. 518–524.2. Drago F, Ciccarese G, Rebora A, Broccolo F, Parodi A. Pityriasis rosea: a comprehensive classification. Dermatology. 2016; 232(4):431–437. PMID: 27096928.
Article3. Chuh A, Zawar V, Lee A. Atypical presentations of pityriasis rosea: case presentations. J Eur Acad Dermatol Venereol. 2005; 19(1):120–126. PMID: 15649208.
Article4. Worldometer. Total coronavirus cases in South Korea. Updated 2022. Accessed February 24, 2022. https://www.worldometers.info/coronavirus/country/south-korea/ .5. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020; 324(8):782–793. PMID: 32648899.
Article6. Shin HS, Park H, Kwon JS, Namgoong H, Kim SJ, Kim JM, et al. National Academy of Medicine of Korea (NAMOK) key statements on COVID-19. J Korean Med Sci. 2021; 36(41):e287. PMID: 34697930.
Article7. Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ, et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020; 395(10242):1973–1987. PMID: 32497510.
Article8. Chiu NC, Chi H, Tai YL, Peng CC, Tseng CY, Chen CC, et al. Impact of wearing masks, hand hygiene, and social distancing on influenza, enterovirus, and all-cause pneumonia during the coronavirus pandemic: retrospective national epidemiological surveillance study. J Med Internet Res. 2020; 22(8):e21257. PMID: 32750008.
Article9. Tanislav C, Kostev K. Fewer non-COVID-19 respiratory tract infections and gastrointestinal infections during the COVID-19 pandemic. J Med Virol. 2022; 94(1):298–302. PMID: 34491581.
Article10. Yoo IH, Kang HM, Jeong DC. Changes in the incidence of intussusception and infectious diseases after the COVID-19 pandemic in Korea. J Korean Med Sci. 2022; 37(8):e60. PMID: 35226418.
Article11. Choe YJ, Lee Y, Shim JO. Impact of social distancing on intussusception incidence in children. J Korean Med Sci. 2022; 37(2):e16. PMID: 35014228.
Article12. Ahn SY, Park JY, Lim IS, Chae SA, Yun SW, Lee NM, et al. Changes in the occurrence of gastrointestinal infections after COVID-19 in Korea. J Korean Med Sci. 2021; 36(24):e180. PMID: 34155841.
Article13. Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020; 33(4):e13730. PMID: 32475003.
Article14. Galván Casas C, Català A, Carretero Hernández G, Rodríguez-Jiménez P, Fernández-Nieto D, Rodríguez-Villa Lario A, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020; 183(1):71–77. PMID: 32348545.
Article15. Wollina U, Karadağ AS, Rowland-Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020; 33(5):e13549. PMID: 32390279.16. Grieco T, Maddalena P, Sernicola A, Muharremi R, Basili S, Alvaro D, et al. Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: an observational study. Dermatol Ther. 2021; 34(6):e15153. PMID: 34622531.
Article17. McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021; 85(1):46–55. PMID: 33838206.
Article18. Tihy M, Menzinger S, André R, Laffitte E, Toutous-Trellu L, Kaya G. Clinicopathological features of cutaneous reactions after mRNA-based COVID-19 vaccines. J Eur Acad Dermatol Venereol. 2021; 35(12):2456–2461. PMID: 34459036.
Article19. McMahon DE, Kovarik CL, Damsky W, Rosenbach M, Lipoff JB, Tyagi A, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022; 86(1):113–121. PMID: 34517079.
Article20. Drago F, Ciccarese G, Rebora A, Parodi A. Human herpesvirus-6, -7, and Epstein-Barr virus reactivation in pityriasis rosea during COVID-19. J Med Virol. 2021; 93(4):1850–1851. PMID: 32970319.
Article21. Watanabe T, Kawamura T, Jacob SE, Aquilino EA, Orenstein JM, Black JB, et al. Pityriasis rosea is associated with systemic active infection with both human herpesvirus-7 and human herpesvirus-6. J Invest Dermatol. 2002; 119(4):793–797. PMID: 12406322.
Article22. Martora F, Picone V, Fornaro L, Fabbrocini G, Marasca C. Can COVID-19 cause atypical forms of pityriasis rosea refractory to conventional therapies? J Med Virol. 2022; 94(4):1292–1293. PMID: 34931329.
Article23. Carballido Vázquez AM, Morgado B. Pityriasis rosea-like eruption after Pfizer-BioNTech COVID-19 vaccination. Br J Dermatol. 2021; 185(2):e34. PMID: 33904157.
Article24. Larson V, Seidenberg R, Caplan A, Brinster NK, Meehan SA, Kim RH. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J Cutan Pathol. 2022; 49(1):34–41. PMID: 34292611.
Article25. Drago F, Broccolo F, Rebora A. Pityriasis rosea: an update with a critical appraisal of its possible herpesviral etiology. J Am Acad Dermatol. 2009; 61(2):303–318. PMID: 19615540.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comments to "A Case of Pityriasis Rosea Associated with Leuprolide Acetate"
- Effect of UVB Phototherapy on Pruritus of Pityriasis Rosea
- A Case of Pityriasis Rosea Associated with Leuprolide Acetate
- Atypical Pityriasis Rosea with Palmoplantar Involvement
- Clinical and Histologic Features of Pityriasis Rosea and Pityriasis Lichenoides in Children