J Korean Med Sci.
2022 Jun;37(22):e183. 10.3346/jkms.2022.37.e183.
A Case of Pathologically Confirmed Corticobasal Degeneration Initially Presenting as Progressive Supranuclear Palsy Syndrome
- Affiliations
-
- 1Department of Neurology, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- 2Inje University Busan Paik Hospital Brain Bank, Busan, Korea
- 3Dementia and Neurodegenerative Disease Research Center, Inje University, Busan, Korea
- 4Department of Pathology, Busan Paik Hospital, Inje University College of Medicine, Busan, Korea
- 5Department of Neurology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea
- 6Department of Anatomy, Inje University College of Medicine, Busan, Korea
- 7Department of Pathology, BioMedical Sciences Graduate Program (BMSGP), Chonnam National University Hwasun Hospital and Medical School, Hwasun, Korea
- 8Chonnam National University Hospital Brain Bank, Gwangju, Korea
- KMID: 2530421
- DOI: http://doi.org/10.3346/jkms.2022.37.e183
Abstract
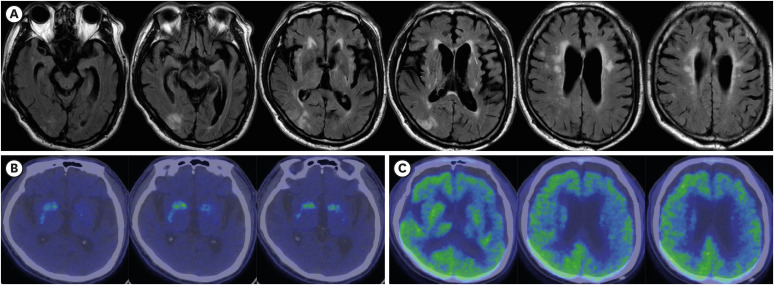
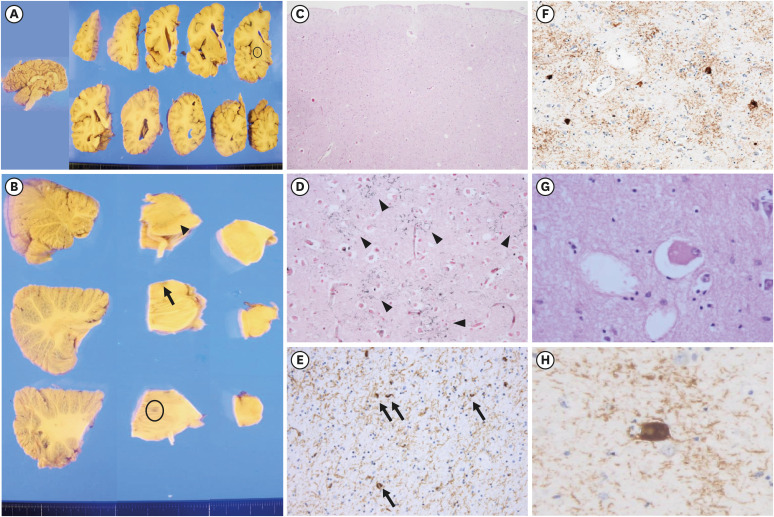
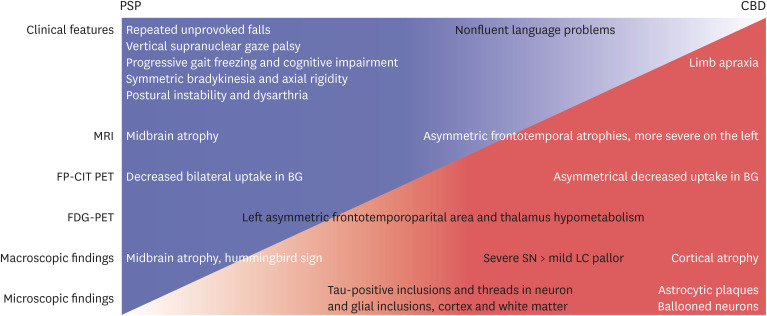
- Progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) overlap clinically with parkinsonism or extrapyramidal signs and pathologically with tauopathy. Asymmetric parkinsonism and cortical dysfunctions are classical features of CBD. However, symmetric parkinsonism, frequent falls, and supranuclear gaze palsy are key features of PSP. Despite biochemically classified as 4R tauopathies, tufted astrocytes of PSP and astrocytic plaque of CBD show pathologically important differences. Herein, we report a 68-year-old man with pathologically confirmed CBD. He was clinically suspected to have PSP because of progressive gait disturbances, frequent falls, and vertical saccade limitation. Neurological examination performed at age 71 revealed symmetrical bradykinesia, axial rigidity, and postural instability with worsening of early existing symptoms. Magnetic resonance imaging of the brain taken at age 70 detected midbrain and left frontotemporal atrophy and right middle cerebral artery infarction. Left frontotemporoparietal hypometabolism and asymmetrically decreased fluoro-propyl-carbomethoxy-iodophenyl-tropane uptake in the basal ganglia were observed. The autopsy was performed at the time of his death (at age 72), which revealed severe pallor of the substantia nigra and mildly hypopigmented locus ceruleus. AT8 immunohistochemistry and Gallyas staining revealed tau-positive neuronal and glial inclusions, astrocytic plaques, ballooned neurons, and numerous threads in both gray and white matter. No abnormal inclusions were revealed by beta-amyloid, α-synuclein and TDP-43 immunohistochemistry. In our case, cerebral infarction, periventricular and deep white matter ischemic changes, and midbrain atrophy were likely to produce PSP–CBD overlapping symptoms. However, our patient was finally confirmed to have CBD based on pathological findings such as astrocytic plaques.
Keyword
Figure
Reference
-
1. Tolnay M, Probst A. The neuropathological spectrum of neurodegenerative tauopathies. IUBMB Life. 2003; 55(6):299–305. PMID: 12938731.2. Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013; 80(5):496–503. PMID: 23359374.3. Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017; 32(6):853–864. PMID: 28467028.4. Rösler TW, Tayaranian Marvian A, Brendel M, Nykänen NP, Höllerhage M, Schwarz SC, et al. Four-repeat tauopathies. Prog Neurobiol. 2019; 180:101644. PMID: 31238088.5. Irwin DJ. Tauopathies as clinicopathological entities. Parkinsonism Relat Disord. 2016; 22(Suppl 1):S29–S33. PMID: 26382841.6. Yoshida M. Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration. Neuropathology. 2014; 34(6):555–570. PMID: 25124031.7. Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002; 61(11):935–946. PMID: 12430710.8. Ling H, O’Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010; 133(Pt 7):2045–2057. PMID: 20584946.9. Tsuboi Y, Josephs KA, Boeve BF, Litvan I, Caselli RJ, Caviness JN, et al. Increased tau burden in the cortices of progressive supranuclear palsy presenting with corticobasal syndrome. Mov Disord. 2005; 20(8):982–988. PMID: 15834857.10. Lee KH, Seo SW, Lim TS, Kim EJ, Kim BC, Kim Y, et al. Proposal guidelines for standardized operating procedures of brain autopsy: Brain bank in South Korea. Yonsei Med J. 2017; 58(5):1055–1060. PMID: 28792154.11. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991; 41(4):479–486. PMID: 2011243.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Corticobasal Degeneration Presenting as Non-Fluent/Agrammatic Primary Progressive Aphasia: A Case Report
- Probable Creutzfeldt-Jakob Disease Presenting as Progressive Supranuclear Palsy
- PET studies in Alzheimer Disease and Other Degenerative Dementias
- Longitudinal Clinical Changes of Non-Fluent/Agrammatic Primary Progressive Aphasia as Tau Spectrum Disorder: A Case Report
- Progressive Supranuclear Palsy with Predominant Cerebellar Ataxia