Korean J Sports Med.
2022 Jun;40(2):67-85. 10.5763/kjsm.2022.40.2.67.
The Mechanisms of Anabolic Steroids, Selective Androgen Receptor Modulators and Myostatin Inhibitors
- Affiliations
-
- 1Department of Exercise Science, David B. Falk College of Sport and Human Dynamics, Syracuse University, Syracuse, NY, USA
- 2Department of Biology, College of Arts and Sciences, Syracuse University, Syracuse, NY, USA
- KMID: 2530310
- DOI: http://doi.org/10.5763/kjsm.2022.40.2.67
Abstract
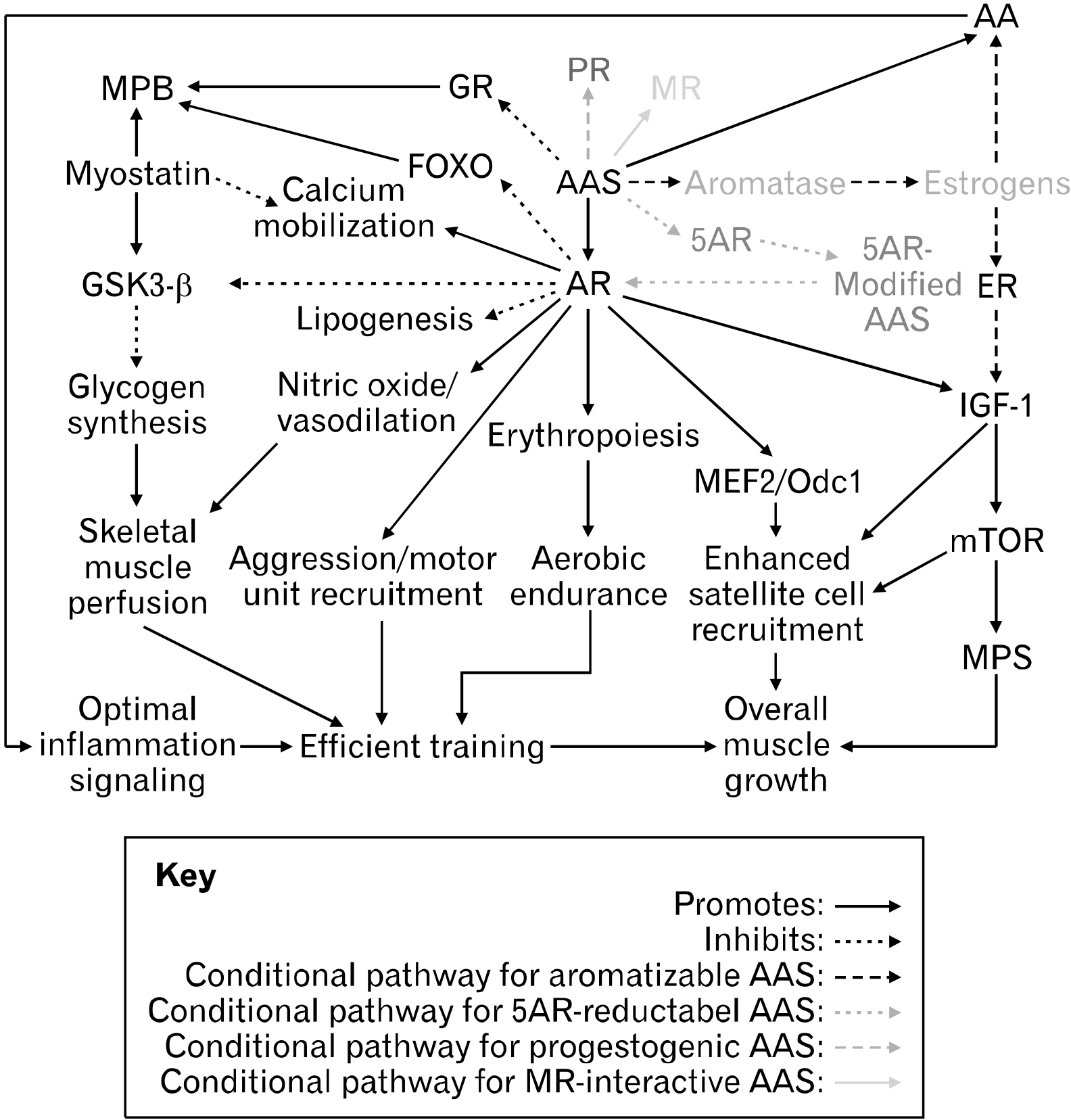
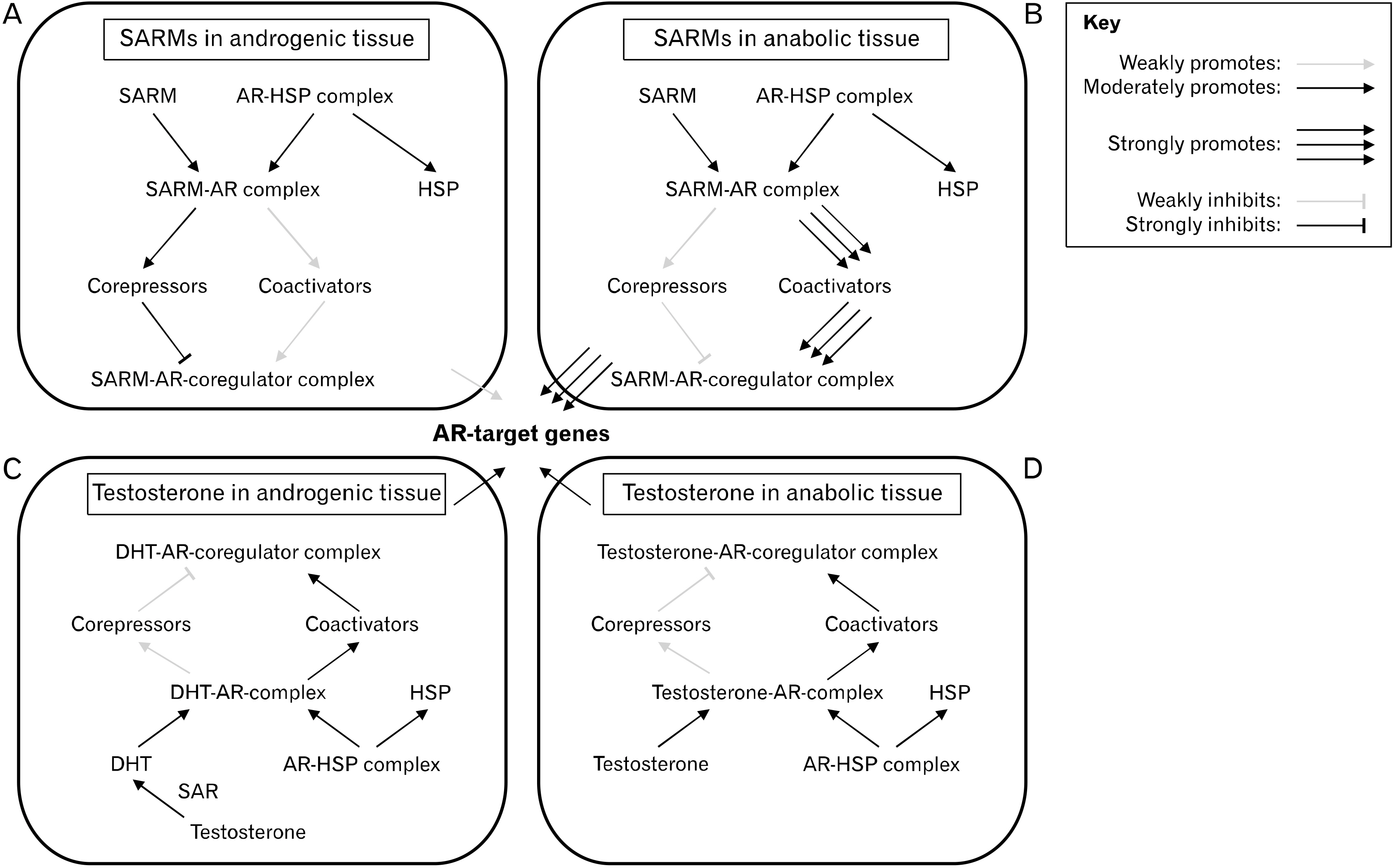
- In the clinical setting, anabolic agents serve to ameliorate muscle- and bone-wasting diseases. However, many of these anabolic agents are also used by bodybuilders to surpass natural limits of body composition as performanceenhancing drugs (PEDs). The first generation of PEDs comprises testosterone-derived anabolic-androgenic steroids (AAS) which have demonstrated significant myotropic effects. However, AAS lack optimal tissue-selectively and thus, are prone to numerous adverse health consequences. Hence, a newer generation of PEDs, selective androgen receptor modulators (SARMs), was developed with the goal of achieving superior tissue-selectivity (i.e., exerting anabolic effects only in muscle and bone tissue, while minimally affecting other body systems). In general, AAS and SARMs enhance muscle growth primarily through androgen receptor (AR) agonism in target tissues. Despite multiple attempts, no single AAS nor SARM to date is completely risk free. As such, a significant portion of research efforts has been dedicated to manipulating anabolic pathways beyond the AR. Another class of PEDs, myostatin inhibitors, have shown to cause drastic muscle anabolism across multiple species by inhibiting myostatin, the primary deterrent to continuous muscle growth. The myostatin inhibitor, YK-11, blocks myostatin by upregulating its antagonist, follistatin. This effect appears to be mediated through the AR, suggesting a novel and promising gene-selective approach to engineering AR ligands that isolate benefits from risks. At any rate, the exact mechanisms by which these PEDs function is not well understood. Further pioneering regarding these topics is encouraged as it appears that the innovation of a truly tissue-selective anabolic agent is within reach.
Keyword
Figure
Cited by 1 articles
-
저항성 운동과 근골격 성장의 생리
Jeremy Park, Vera Mcllvain, Jared Rosenberg, Lorin Donovan, Priya Desai, Joon Young Kim
Korean J Sports Med. 2022;40(3):151-169. doi: 10.5763/kjsm.2022.40.3.151.
Reference
-
1. Aragon AA, Schoenfeld BJ, Wildman R, et al. 2017; International society of sports nutrition position stand: diets and body composition. J Int Soc Sports Nutr. 14:16. DOI: 10.1186/s12970-017-0174-y. PMID: 28630601. PMCID: PMC5470183.
Article2. Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA. 2015; Satellite cells and skeletal muscle regeneration. Compr Physiol. 5:1027–59. DOI: 10.1002/cphy.c140068. PMID: 26140708.
Article3. Kraemer WJ, Ratamess NA. 2005; Hormonal responses and adaptations to resistance exercise and training. Sports Med. 35:339–61. DOI: 10.2165/00007256-200535040-00004. PMID: 15831061.
Article4. Schoenfeld BJ. 2010; The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 24:2857–72. DOI: 10.1519/JSC.0b013e3181e840f3. PMID: 20847704.
Article5. Elliott B, Renshaw D, Getting S, Mackenzie R. 2012; The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf). 205:324–40. DOI: 10.1111/j.1748-1716.2012.02423.x. PMID: 22340904.
Article6. Kouri EM, Pope HG Jr, Katz DL, Oliva P. 1995; Fat-free mass index in users and nonusers of anabolic-androgenic steroids. Clin J Sport Med. 5:223–8. DOI: 10.1097/00042752-199510000-00003. PMID: 7496846.
Article7. Shahidi NT. 2001; A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther. 23:1355–90. DOI: 10.1016/S0149-2918(01)80114-4. PMID: 11589254.
Article8. Bonnecaze AK, O'Connor T, Burns CA. 2021; Harm reduction in male patients actively using anabolic androgenic steroids (AAS) and performance-enhancing drugs (PEDs): a review. J Gen Intern Med. 36:2055–64. DOI: 10.1007/s11606-021-06751-3. PMID: 33948794. PMCID: PMC8298654.
Article9. Rahman F, Christian HC. 2007; Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 18:371–8. DOI: 10.1016/j.tem.2007.09.004. PMID: 17997105.
Article10. Lipsett MB. 1975; Production of testosterone by prostate and other peripheral tissues in man. Vitam Horm. 33:209–21. DOI: 10.1016/S0083-6729(08)60957-7. PMID: 180673.
Article11. Stocco DM, Clark BJ. 1996; Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 17:221–44. DOI: 10.1210/edrv-17-3-221. PMID: 8771357.
Article12. Midzak AS, Chen H, Papadopoulos V, Zirkin BR. 2009; Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 299:23–31. DOI: 10.1016/j.mce.2008.07.016. PMID: 18761053.
Article13. Pastuszak AW, Gittelman M, Tursi JP, Jaffe JS, Schofield D, Miner MM. 2022; Pharmacokinetics of testosterone therapies in relation to diurnal variation of serum testosterone levels as men age. Andrology. 10:209–22. DOI: 10.1111/andr.13108. PMID: 34510812.
Article14. Llewellyn W. William Llewellyn's anabolics. Molecular Nutrition LLC;Jupiter (FL): 11th ed. 2017.15. Mottram DR, George AJ. 2000; Anabolic steroids. Baillieres Best Pract Res Clin Endocrinol Metab. 14:55–69. DOI: 10.1053/beem.2000.0053. PMID: 10932810.
Article16. Rahnema CD, Lipshultz LI, Crosnoe LE, Kovac JR, Kim ED. 2014; Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril. 101:1271–9. DOI: 10.1016/j.fertnstert.2014.02.002. PMID: 24636400.
Article17. Pan MM, Kovac JR. 2016; Beyond testosterone cypionate: evidence behind the use of nandrolone in male health and wellness. Transl Androl Urol. 5:213–9. DOI: 10.21037/tau.2016.03.03. PMID: 27141449. PMCID: PMC4837307.
Article18. Basualto-Alarcón C, Jorquera G, Altamirano F, Jaimovich E, Estrada M. 2013; Testosterone signals through mTOR and androgen receptor to induce muscle hypertrophy. Med Sci Sports Exerc. 45:1712–20. DOI: 10.1249/MSS.0b013e31828cf5f3. PMID: 23470307.
Article19. Yarrow JF, McCoy SC, Borst SE. 2010; Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): a potent anabolic steroid with reduced androgenic and estrogenic activity. Steroids. 75:377–89. DOI: 10.1016/j.steroids.2010.01.019. PMID: 20138077.
Article20. Haren MT, Siddiqui AM, Armbrecht HJ, et al. 2011; Testosterone modulates gene expression pathways regulating nutrient accumulation, glucose metabolism and protein turnover in mouse skeletal muscle. Int J Androl. 34:55–68. DOI: 10.1111/j.1365-2605.2010.01061.x. PMID: 20403060.
Article21. Deane CS, Hughes DC, Sculthorpe N, Lewis MP, Stewart CE, Sharples AP. 2013; Impaired hypertrophy in myoblasts is improved with testosterone administration. J Steroid Biochem Mol Biol. 138:152–61. DOI: 10.1016/j.jsbmb.2013.05.005. PMID: 23714396.
Article22. Braga M, Bhasin S, Jasuja R, Pervin S, Singh R. 2012; Testosterone inhibits transforming growth factor-β signaling during myogenic differentiation and proliferation of mouse satellite cells: potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol. 350:39–52. DOI: 10.1016/j.mce.2011.11.019. PMID: 22138414. PMCID: PMC3264813.
Article23. Serra C, Bhasin S, Tangherlini F, et al. 2011; The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology. 152:193–206. DOI: 10.1210/en.2010-0802. PMID: 21084444. PMCID: PMC3033058.
Article24. Wu Y, Bauman WA, Blitzer RD, Cardozo C. 2010; Testosterone-induced hypertrophy of L6 myoblasts is dependent upon Erk and mTOR. Biochem Biophys Res Commun. 400:679–83. DOI: 10.1016/j.bbrc.2010.08.127. PMID: 20816664.
Article25. Dubois V, Laurent MR, Sinnesael M, et al. 2014; A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. FASEB J. 28:2979–94. DOI: 10.1096/fj.14-249748. PMID: 24671706.
Article26. MacKrell JG, Yaden BC, Bullock H, et al. 2015; Molecular targets of androgen signaling that characterize skeletal muscle recovery and regeneration. Nucl Recept Signal. 13:e005. DOI: 10.1621/nrs.13005. PMID: 26457071. PMCID: PMC4599140.
Article27. Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. 2004; Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 89:5245–55. DOI: 10.1210/jc.2004-0084. PMID: 15472231.
Article28. Lucas-Herald AK, Alves-Lopes R, Montezano AC, Ahmed SF, Touyz RM. 2017; Genomic and non-genomic effects of androgens in the cardiovascular system: clinical implications. Clin Sci (Lond). 131:1405–18. DOI: 10.1042/CS20170090. PMID: 28645930. PMCID: PMC5736922.
Article29. O'Malley BW, Tsai SY, Bagchi M, Weigel NL, Schrader WT, Tsai MJ. 1991; Molecular mechanism of action of a steroid hormone receptor. Recent Prog Horm Res. 47:1–24. DOI: 10.1016/B978-0-12-571147-0.50005-6. PMID: 1745818.30. Wärnmark A, Treuter E, Wright AP, Gustafsson JA. 2003; Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation. Mol Endocrinol. 17:1901–9. DOI: 10.1210/me.2002-0384. PMID: 12893880.
Article31. Skinner MK. Encyclopedia of reproduction. Elsevier, Academic Press;Amsterdam, Boston:32. Narayanan R, Coss CC, Dalton JT. 2018; Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol. 465:134–42. DOI: 10.1016/j.mce.2017.06.013. PMID: 28624515. PMCID: PMC5896569.
Article33. Bevan CL, Hoare S, Claessens F, Heery DM, Parker MG. 1999; The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1. Mol Cell Biol. 19:8383–92. DOI: 10.1128/MCB.19.12.8383. PMID: 10567563. PMCID: PMC84931.
Article34. Singh R, Bhasin S, Braga M, et al. 2009; Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 150:1259–68. DOI: 10.1210/en.2008-0858. PMID: 18948405. PMCID: PMC2654730.35. Parr MK, Müller-Schöll A. 2018; Pharmacology of doping agents— mechanisms promoting muscle hypertrophy. AIMS Mol Sci. 5:145–55. DOI: 10.3934/molsci.2018.2.131.36. Rana K, Lee NK, Zajac JD, MacLean HE. 2014; Expression of androgen receptor target genes in skeletal muscle. Asian J Androl. 16:675–83. DOI: 10.4103/1008-682X.122861. PMID: 24713826. PMCID: PMC4215656.
Article37. Wyce A, Bai Y, Nagpal S, Thompson CC. 2010; Research resource: the androgen receptor modulates expression of genes with critical roles in muscle development and function. Mol Endocrinol. 24:1665–74. DOI: 10.1210/me.2010-0138. PMID: 20610535. PMCID: PMC5417449.
Article38. Liu N, Nelson BR, Bezprozvannaya S, et al. 2014; Requirement of MEF2A, C, and D for skeletal muscle regeneration. Proc Natl Acad Sci U S A. 111:4109–14. DOI: 10.1073/pnas.1401732111. PMID: 24591619. PMCID: PMC3964114.
Article39. Lee NK, Skinner JP, Zajac JD, MacLean HE. 2011; Ornithine decarboxylase is upregulated by the androgen receptor in skeletal muscle and regulates myoblast proliferation. Am J Physiol Endocrinol Metab. 301:E172–9. DOI: 10.1152/ajpendo.00094.2011. PMID: 21505150.
Article40. Saartok T, Dahlberg E, Gustafsson JA. 1984; Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 114:2100–6. DOI: 10.1210/endo-114-6-2100. PMID: 6539197.
Article41. Wu FC. 1997; Endocrine aspects of anabolic steroids. Clin Chem. 43:1289–92. DOI: 10.1093/clinchem/43.7.1289. PMID: 9216476.
Article42. Hartgens F, Kuipers H. 2004; Effects of androgenic-anabolic steroids in athletes. Sports Med. 34:513–54. DOI: 10.2165/00007256-200434080-00003. PMID: 15248788.
Article43. El-Maouche D, Dobs A. Legato MJ, editor. Chapter 60. Testosterone replacement therapy in men and women. Principles of gender-specific medicine. Academic Press;San Diego: 2nd ed. 2010. p. 737–60. DOI: 10.1016/B978-0-12-374271-1.00060-5.44. Sheffield-Moore M. 2000; Androgens and the control of skeletal muscle protein synthesis. Ann Med. 32:181–6. DOI: 10.3109/07853890008998825. PMID: 10821325.
Article45. Foryst-Ludwig A, Kintscher U. 2010; Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 122:74–81. DOI: 10.1016/j.jsbmb.2010.06.012. PMID: 20599505.
Article46. Dayton WR, White ME. 2014; Meat Science and Muscle Biology Symposium: role of satellite cells in anabolic steroid-induced muscle growth in feedlot steers. J Anim Sci. 92:30–8. DOI: 10.2527/jas.2013-7077. PMID: 24166993.47. Kahlert S, Grohé C, Karas RH, Löbbert K, Neyses L, Vetter H. 1997; Effects of estrogen on skeletal myoblast growth. Biochem Biophys Res Commun. 232:373–8. DOI: 10.1006/bbrc.1997.6223. PMID: 9125184.
Article48. Balgoma D, Zelleroth S, Grönbladh A, Hallberg M, Pettersson C, Hedeland M. 2020; Anabolic androgenic steroids exert a selective remodeling of the plasma lipidome that mirrors the decrease of the de novo lipogenesis in the liver. Metabolomics. 16:12. DOI: 10.1007/s11306-019-1632-0. PMID: 31925559. PMCID: PMC6954146.
Article49. Labrie F, Luu-The V, Labrie C, Simard J. 2001; DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 22:185–212. DOI: 10.1006/frne.2001.0216. PMID: 11456468.
Article50. Markworth JF, Cameron-Smith D. 2013; Arachidonic acid supplementation enhances in vitro skeletal muscle cell growth via a COX-2-dependent pathway. Am J Physiol Cell Physiol. 304:C56–67. DOI: 10.1152/ajpcell.00038.2012. PMID: 23076795.
Article51. Hiipakka RA, Liao S. 1998; Molecular mechanism of androgen action. Trends Endocrinol Metab. 9:317–24. DOI: 10.1016/S1043-2760(98)00081-2. PMID: 18406296.
Article52. van Amsterdam J, Opperhuizen A, Hartgens F. 2010; Adverse health effects of anabolic-androgenic steroids. Regul Toxicol Pharmacol. 57:117–23. DOI: 10.1016/j.yrtph.2010.02.001. PMID: 20153798.
Article53. Kicman AT. 2008; Pharmacology of anabolic steroids. Br J Pharmacol. 154:502–21. DOI: 10.1038/bjp.2008.165. PMID: 18500378. PMCID: PMC2439524.
Article54. Suvitha A, Souissi M, Sahara R, Venkataramanan NS. 2019; Deciphering the nature of interactions in nandrolone/testosterone encapsulated cucurbituril complexes : a computational study. J Incl Phenom Macrocycl Chem. 93:183–92. DOI: 10.1007/s10847-018-0869-y.
Article55. Pomara C, Barone R, Marino Gammazza A, et al. 2016; Effects of nandrolone stimulation on testosterone biosynthesis in Leydig cells. J Cell Physiol. 231:1385–91. DOI: 10.1002/jcp.25272. PMID: 26626779. PMCID: PMC5064776.
Article56. Chang MC, Hafez ES, Merrill A, Pincus G, Zarrow MX. 1956; Studies of the biological activity of certain 19-nor steroids in female animals. Endocrinology. 59:695–707. DOI: 10.1210/endo-59-6-695. PMID: 13375596.
Article57. Smith GI, Mittendorfer B. 2016; Sexual dimorphism in skeletal muscle protein turnover. J Appl Physiol (1985). 120:674–82. DOI: 10.1152/japplphysiol.00625.2015. PMID: 26702024. PMCID: PMC4796180.
Article58. Wang HQ, Takebayashi K, Tsuchida K, Nishimura M, Noda Y. 2003; Follistatin-related gene (FLRG) expression in human endometrium: sex steroid hormones regulate the expression of FLRG in cultured human endometrial stromal cells. J Clin Endocrinol Metab. 88:4432–9. DOI: 10.1210/jc.2002-021758. PMID: 12970321.
Article59. Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S. 2014; Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction. BMC Clin Pathol. 14:33. DOI: 10.1186/1472-6890-14-33. PMID: 25071417. PMCID: PMC4112981.
Article60. Roberts AC, McClure RD, Weiner RI, Brooks GA. 1993; Overtraining affects male reproductive status. Fertil Steril. 60:686–92. DOI: 10.1016/S0015-0282(16)56223-2. PMID: 8405526.
Article61. Takeda AN, Pinon GM, Bens M, Fagart J, Rafestin-Oblin ME, Vandewalle A. 2007; The synthetic androgen methyltrienolone (r1881) acts as a potent antagonist of the mineralocorticoid receptor. Mol Pharmacol. 71:473–82. DOI: 10.1124/mol.106.031112. PMID: 17105867.
Article62. Houtman CJ, Sterk SS, van de Heijning MP, et al. 2009; Detection of anabolic androgenic steroid abuse in doping control using mammalian reporter gene bioassays. Anal Chim Acta. 637:247–58. DOI: 10.1016/j.aca.2008.09.037. PMID: 19286037.
Article63. Hershberger LG, Shipley EG, Meyer RK. 1953; Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method. Proc Soc Exp Biol Med. 83:175–80. DOI: 10.3181/00379727-83-20301. PMID: 13064212.
Article64. Kam PC, Yarrow M. 2005; Anabolic steroid abuse: physiological and anaesthetic considerations. Anaesthesia. 60:685–92. DOI: 10.1111/j.1365-2044.2005.04218.x. PMID: 15960720.
Article65. National Center for Biotechnology Information (NCBI). 2022. Pub-Chem compound summary for CID 5281034, Oxymetholone [Internet]. NCBI;Bethesda, MD: Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Oxymetholone. cited 2022 May 16.66. Gao W, Dalton JT. 2007; Expanding the therapeutic use of androgens via selective androgen receptor modulators (SARMs). Drug Discov Today. 12:241–8. DOI: 10.1016/j.drudis.2007.01.003. PMID: 17331889. PMCID: PMC2072879.
Article67. Naafs MA. Selective androgen receptor modulators (SARMs): a mini-review. DOI: 10.32474/OAJRSD.2018.01.000103. 2018; 1:20–6.
Article68. Bhasin S, Jasuja R. 2009; Selective androgen receptor modulators as function promoting therapies. Curr Opin Clin Nutr Metab Care. 12:232–40. DOI: 10.1097/MCO.0b013e32832a3d79. PMID: 19357508. PMCID: PMC2907129.
Article69. Zilbermint MF, Dobs AS. 2009; Nonsteroidal selective androgen receptor modulator Ostarine in cancer cachexia. Future Oncol. 5:1211–20. DOI: 10.2217/fon.09.106. PMID: 19852734.70. Basaria S, Collins L, Dillon EL, et al. 2013; The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 68:87–95. DOI: 10.1093/gerona/gls078. PMID: 22459616. PMCID: PMC4111291.
Article71. Sieck GC, Mantilla CB. Miller VM, Hay M, editors. Influence of sex hormones on the neuromuscular junction. Principles of sex-based differences in physiology. Elsevier;Amsterdam, Boston, Heidelberg: 2004. p. 183–94. DOI: 10.1016/S1569-2558(03)34013-5.
Article72. Cunningham RL, Giuffrida A, Roberts JL. 2009; Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cdelta. Endocrinology. 150:5539–48. DOI: 10.1210/en.2009-0640. PMID: 19837873. PMCID: PMC2795716.
Article73. Evans PJ, Lynch RM. 2003; Insulin as a drug of abuse in body building. Br J Sports Med. 37:356–7. DOI: 10.1136/bjsm.37.4.356. PMID: 12893725. PMCID: PMC1724679.
Article74. Graham MR, Evans P, Davies B, Baker JS. 2008; AAS, growth hormone, and insulin abuse: psychological and neuroendocrine effects. Ther Clin Risk Manag. 4:587–97. DOI: 10.2147/TCRM.S2495. PMID: 18827854. PMCID: PMC2500251.
Article75. Mohler ML, Bohl CE, Jones A, et al. 2009; Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J Med Chem. 52:3597–617. DOI: 10.1021/jm900280m. PMID: 19432422.
Article76. Patel K, Amthor H. 2005; The function of Myostatin and strategies of Myostatin blockade-new hope for therapies aimed at promoting growth of skeletal muscle. Neuromuscul Disord. 15:117–26. DOI: 10.1016/j.nmd.2004.10.018. PMID: 15694133.
Article77. Amthor H, Macharia R, Navarrete R, et al. 2007; Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci U S A. 104:1835–40. DOI: 10.1073/pnas.0604893104. PMID: 17267614. PMCID: PMC1794294.
Article78. Mosler S, Geisler S, Hengevoss J, et al. 2013; Modulation of follistatin and myostatin propeptide by anabolic steroids and gender. Int J Sports Med. 34:567–72. DOI: 10.1055/s-0032-1312585. PMID: 23559411.
Article79. Dalbo VJ, Roberts MD, Mobley CB, et al. 2017; Testosterone and trenbolone enanthate increase mature myostatin protein expression despite increasing skeletal muscle hypertrophy and satellite cell number in rodent muscle. Andrologia. 49:10.1111/and.12622. DOI: 10.1111/and.12622. PMID: 27246614.
Article80. Rahimov F, King OD, Warsing LC, et al. 2011; Gene expression profiling of skeletal muscles treated with a soluble activin type IIB receptor. Physiol Genomics. 43:398–407. DOI: 10.1152/physiolgenomics.00223.2010. PMID: 21266502. PMCID: PMC3092338.
Article81. Thevis M, Piper T, Dib J, et al. 2017; Mass spectrometric characterization of the selective androgen receptor modulator (SARM) YK-11 for doping control purposes. Rapid Commun Mass Spectrom. 31:1175–83. DOI: 10.1002/rcm.7886. PMID: 28440570.
Article82. Yatsu T, Kusakabe T, Kato K, Inouye Y, Nemoto K, Kanno Y. 2018; Selective androgen receptor modulator, YK11, up-regulates osteoblastic proliferation and differentiation in MC3T3-E1 cells. Biol Pharm Bull. 41:394–8. DOI: 10.1248/bpb.b17-00748. PMID: 29491216.
Article83. Kanno Y, Ota R, Someya K, Kusakabe T, Kato K, Inouye Y. 2013; Selective androgen receptor modulator, YK11, regulates myogenic differentiation of C2C12 myoblasts by follistatin expression. Biol Pharm Bull. 36:1460–5. DOI: 10.1248/bpb.b13-00231. PMID: 23995658.
Article84. Lee SJ, Gharbi A, Shin JE, Jung ID, Park YM. 2021; Myostatin inhibitor YK11 as a preventative health supplement for bacterial sepsis. Biochem Biophys Res Commun. 543:1–7. DOI: 10.1016/j.bbrc.2021.01.030. PMID: 33588136.
Article85. Kanno Y, Hikosaka R, Zhang SY, et al. 2011; (17α,20E)-17,20-[(1-methoxyethylidene) bis(oxy)]-3-oxo-19-norpregna-4,20-diene-21-carboxylic acid methyl ester (YK11) is a partial agonist of the androgen receptor. Biol Pharm Bull. 34:318–23. DOI: 10.1248/bpb.34.318. PMID: 21372378.86. Suh J, Lee YS. 2020; Myostatin inhibitors: panacea or predicament for musculoskeletal disorders? J Bone Metab. 27:151–65. DOI: 10.11005/jbm.2020.27.3.151. PMID: 32911580. PMCID: PMC7571243.
Article87. Ying SY, Becker A, Swanson G, et al. 1987; Follistatin specifically inhibits pituitary follicle stimulating hormone release in vitro. Biochem Biophys Res Commun. 149:133–9. DOI: 10.1016/0006-291X(87)91614-7. PMID: 3120723.88. Mendias CL, Lynch EB, Gumucio JP, et al. 2015; Changes in skeletal muscle and tendon structure and function following genetic inactivation of myostatin in rats. J Physiol. 593:2037–52. DOI: 10.1113/jphysiol.2014.287144. PMID: 25640143. PMCID: PMC4405758.
Article89. Mendias CL, Bakhurin KI, Faulkner JA. 2008; Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc Natl Acad Sci U S A. 105:388–93. DOI: 10.1073/pnas.0707069105. PMID: 18162552. PMCID: PMC2224222.
Article90. Chang H, Brown CW, Matzuk MM. 2002; Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 23:787–823. DOI: 10.1210/er.2002-0003. PMID: 12466190.
Article91. Xu X, Zheng L, Yuan Q, et al. 2018; Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 6:2. DOI: 10.1038/s41413-017-0005-4. PMID: 29423331. PMCID: PMC5802812.
Article92. Hoffmann R. 2002; Male androgenetic alopecia. Clin Exp Dermatol. 27:373–82. DOI: 10.1046/j.1365-2230.2002.01086.x. PMID: 12190637.
Article93. Bhasin S, Woodhouse L, Casaburi R, et al. 2001; Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 281:E1172–81. DOI: 10.1152/ajpendo.2001.281.6.E1172. PMID: 11701431.94. Farias MM, Cuevas AM, Rodriguez F. 2011; Set-point theory and obesity. Metab Syndr Relat Disord. 9:85–9. DOI: 10.1089/met.2010.0090. PMID: 21117971.
Article95. Müller MJ, Bosy-Westphal A, Heymsfield SB. 2010; Is there evidence for a set point that regulates human body weight? F1000 Med Rep. 2:59. DOI: 10.3410/M2-59. PMID: 21173874. PMCID: PMC2990627.
Article96. Mueller MJ, Maluf KS. 2002; Tissue adaptation to physical stress: a proposed "Physical Stress Theory" to guide physical therapist practice, education, and research. Phys Ther. 82:383–403. DOI: 10.1093/ptj/82.4.383. PMID: 11922854.
Article97. Bickel CS, Cross JM, Bamman MM. 2011; Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc. 43:1177–87. DOI: 10.1249/MSS.0b013e318207c15d. PMID: 21131862.
Article98. Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. 2010; Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. Proc Natl Acad Sci U S A. 107:15111–6. DOI: 10.1073/pnas.0913935107. PMID: 20713720. PMCID: PMC2930527.
Article99. Seaborne RA, Strauss J, Cocks M, et al. 2018; Methylome of human skeletal muscle after acute & chronic resistance exercise training, detraining & retraining. Sci Data. 5:180213. DOI: 10.1038/sdata.2018.213. PMID: 30375987. PMCID: PMC6207066.
Article100. Schoenfeld BJ, Ogborn D, Krieger JW. 2016; Effects of resistance training frequency on measures of muscle hypertrophy: a systematic review and meta-analysis. Sports Med. 46:1689–97. DOI: 10.1007/s40279-016-0543-8. PMID: 27102172.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in the Drug Therapy of Osteoporosis
- Pattern Alopecia during Hormonal Anticancer Therapy in Patients with Breast Cancer
- A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction
- Analgesic Effects of Antiosteoporotic Drugs
- Current and Upcoming Treatments for Osteoporosis