J Korean Med Sci.
2022 May;37(20):e164. 10.3346/jkms.2022.37.e164.
Latent Tuberculosis Cascade of Care Among Healthcare Workers: A Nationwide Cohort Analysis in Korea Between 2017 and 2018
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Urology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Occupational and Environmental Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University, Seoul, Korea
- 5Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 6Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 7Division of Pulmonary Medicine, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- KMID: 2530107
- DOI: http://doi.org/10.3346/jkms.2022.37.e164
Abstract
- Background
In 2017, Korea implemented nationwide latent tuberculosis infection (LTBI) project targeting healthcare workers (HCWs). We aimed to assess its performance using the cascade of care model.
Methods
We included 45,503 employees of medical institutions with positive interferongamma release assay result who participated between March 2017 and December 2018. We described percentages of LTBI participants completing each step in the cascade of care. Poisson regression model was conducted to assess individual characteristics and factors associated with not-visiting clinics for further care, not-initiating LTBI treatment, and notcompleting treatment.
Results
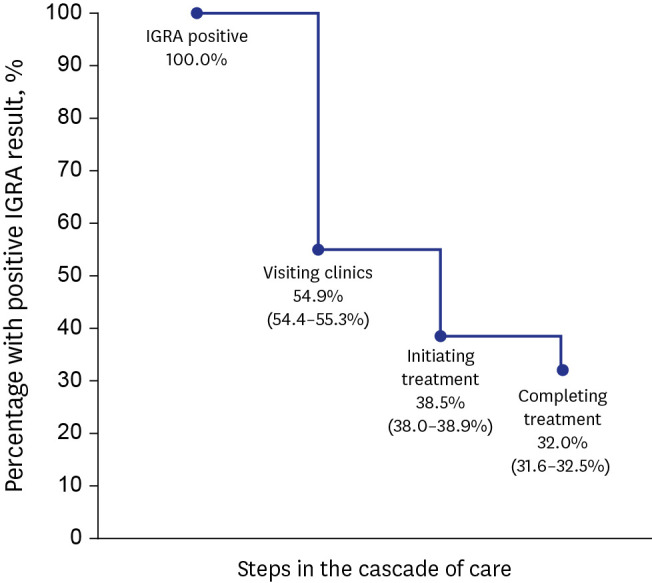
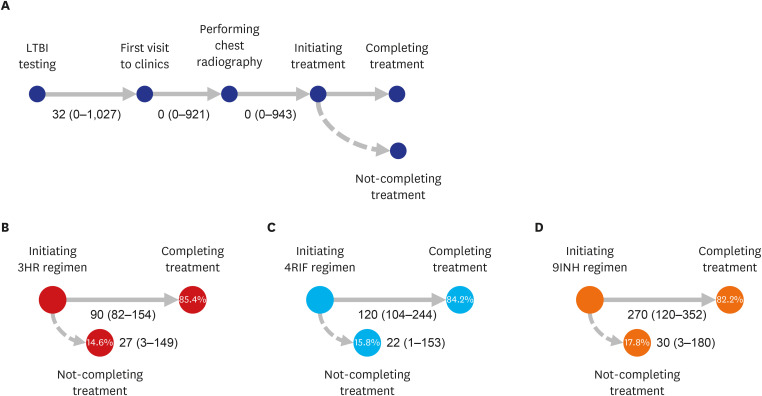
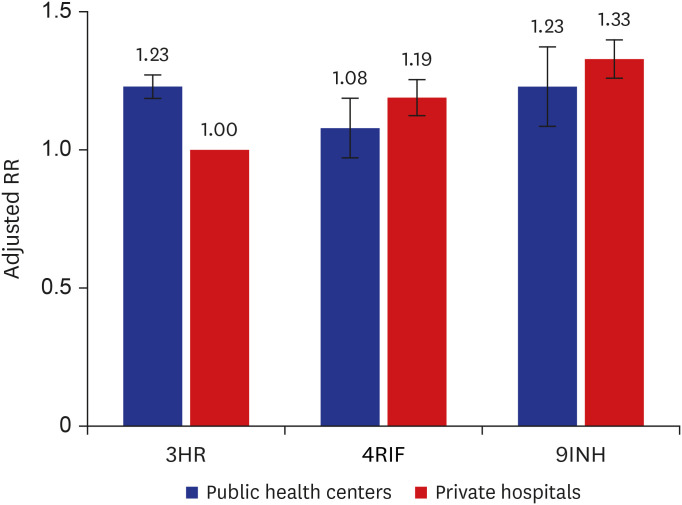
Proportions of visiting clinics and initiating and completing treatment in HCWs were 54.9%, 38.5%, and 32.0%, respectively. Despite of less likelihood of visiting clinics and initiating LTBI treatment, older age ≥ 65 years were more likely to complete treatment (adjusted relative risk [aRR], 0.80; 95% confidence interval [CI], 0.64–0.99), compared to young age < 35 years. Compared to nurses, doctors were less likely to visit clinic; however, were more likely to initiate treatment (aRR, 0.88; 95% CI, 0.81–0.96). Those who visited public health centers were associated with not-initiating treatment (aRR, 1.34; 95% CI, 1.29–1.40). When treated at private hospitals, 9-month isoniazid monotherapy was less likely to complete treatment, compared to 3-month isoniazid and rifampicin combination therapy (aRR, 1.33; 95% CI, 1.16–1.53).
Conclusion
Among employees of medical institutions with LTBI, only one third completed treatment. Age, occupation, treatment center, and initial regimen were significantly related to LTBI treatment performance indicators. Rifampicin-based short treatment regimens were effective under standard of care.
Keyword
Figure
Cited by 1 articles
-
Institutional Tuberculosis Control and Elimination Program
Shi Nae Yu, Tae Hyong Kim, Su Ha Han, Yang-Ki Kim
Korean J Healthc Assoc Infect Control Prev. 2023;28(1):22-28. doi: 10.14192/kjicp.2023.28.1.22.
Reference
-
1. Apriani L, McAllister S, Sharples K, Alisjahbana B, Ruslami R, Hill PC, et al. Latent tuberculosis infection in healthcare workers in low- and middle-income countries: an updated systematic review. Eur Respir J. 2019; 53(4):1801789. PMID: 30792341.2. Uden L, Barber E, Ford N, Cooke GS. Risk of tuberculosis infection and disease for health care workers: an updated meta-analysis. Open Forum Infect Dis. 2017; 4(3):ofx137. PMID: 28875155.3. Mullie GA, Schwartzman K, Zwerling A, N’Diaye DS. Revisiting annual screening for latent tuberculosis infection in healthcare workers: a cost-effectiveness analysis. BMC Med. 2017; 15(1):104. PMID: 28514962.4. Gill J, Prasad V. Testing healthcare workers for latent tuberculosis: Is it evidence based, bio-plausible, both, or neither? Am J Med. 2019; 132(11):1260–1261. PMID: 30946831.5. World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 2: Screening – Systematic Screening for Tuberculosis Disease. Geneva, Switzerland: World Health Organization;2021.6. World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Tuberculosis Preventive Treatment: Module 1: Prevention. Geneva, Switzerland: World Health Organization;2020.7. Ahn JG, Kim DS, Kim KH. Nosocomial exposure to active pulmonary tuberculosis in a neonatal intensive care unit. Am J Infect Control. 2015; 43(12):1292–1295. PMID: 26307044.8. Oh CE, Kwon GY, Kwon YH, Lee EJ, Park MS, Kim SH, et al. High tuberculosis transmission rate in children with nursery exposure to undetected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2018; 22(9):1031–1036. PMID: 30092868.9. Kwon Y, Kim SJ, Kim J, Kim SY, Song EM, Lee EJ, et al. Results of tuberculosis contact investigation in congregate settings in Korea, 2013. Osong Public Health Res Perspect. 2014; 5(Suppl):S30–S36. PMID: 25861578.10. Kim TH, Jang YS, Jung SJ, Kim YJ, Pai HJ, Oh SH. A tuberculosis contact investigation on health care workers in one hospital. Pediatr Infect Vaccine. 2016; 23(2):94–101.11. Jeon D. Latent tuberculosis infection: recent progress and challenges in South Korea. Korean J Intern Med. 2020; 35(2):269–275. PMID: 32131570.12. Go U, Park M, Kim UN, Lee S, Han S, Lee J, et al. Tuberculosis prevention and care in Korea: evolution of policy and practice. J Clin Tuberc Other Mycobact Dis. 2018; 11:28–36. PMID: 31720389.13. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016; 16(11):1269–1278. PMID: 27522233.14. Min J, Kim HW, Stagg HR, Lipman M, Rangaka MX, Myong JP, et al. Latent tuberculosis infection screening and treatment in congregate settings (TB FREE COREA): protocol for a prospective observational study in Korea. BMJ Open. 2020; 10(2):e034098.15. Kim HW, Min J, Choi JY, Shin AY, Myong JP, Lee Y, et al. Latent tuberculosis infection screening and treatment in congregate settings (TB FREE COREA): demographic profiles of interferon-gamma release assay cohort. J Korean Med Sci. 2021; 36(36):e246. PMID: 34519187.16. Min J, Kim JS. Diagnosis and treatment of latent tuberculosis infection. J Korean Med Assoc. 2019; 62(1):11–17.17. Joint Committee for the Revision of Korean Guidelines for Tuberculosis. Korea Centers for Disease Control and Prevention. Korean Guidelines for Tuberculosis. 4th Edition. Osong, Korea: Korea Centers for Disease Control and Prevention;2020.18. Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H, et al. Four months of rifampin or nine months of isoniazid for latent tuberculosis in adults. N Engl J Med. 2018; 379(5):440–453. PMID: 30067931.19. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005; 43(11):1130–1139. PMID: 16224307.20. Subbaraman R, Nathavitharana RR, Mayer KH, Satyanarayana S, Chadha VK, Arinaminpathy N, et al. Constructing care cascades for active tuberculosis: a strategy for program monitoring and identifying gaps in quality of care. PLoS Med. 2019; 16(2):e1002754. PMID: 30811385.21. Lee S, Lee YL, Kim YC, Kim EJ, Heo JY, Choi YH. Prevalence and risk factors of latent tuberculosis infection among healthcare workers. Korean J Healthc Assoc Infect Control Prev. 2019; 24(2):52–59.22. Park SY, Lee E, Lee EJ, Kim TH, Kim YK. Screening and treatment of latent tuberculosis infection among healthcare workers at a referral hospital in Korea. Infect Chemother. 2019; 51(4):355–364. PMID: 31898423.23. Lee JY, Park JY, Kim MS, Kim JH, Lee JY. Selection of health care workers for screening for latent tuberculosis infection. J Tuberc Res. 2019; 7(2):65–76.24. Han SS, Lee SJ, Yim JJ, Song JH, Lee EH, Kang YA. Evaluation and treatment of latent tuberculosis infection among healthcare workers in Korea: a multicentre cohort analysis. PLoS One. 2019; 14(9):e0222810. PMID: 31536577.25. Lee EH, Kim SJ, Ha EJ, Park ES, Choi JY, Leem AY, et al. Treatment of latent tuberculous infection among health care workers at a tertiary hospital in Korea. Int J Tuberc Lung Dis. 2018; 22(11):1336–1343. PMID: 30355414.26. Souza AB, Arriaga MB, Amorim G, Araújo-Pereira M, Nogueira BM, Queiroz AT, et al. Determinants of losses in the latent tuberculosis infection cascade of care in Brazil. BMJ Glob Health. 2021; 6(9):e005969.27. Campbell JR, Dowdy D, Schwartzman K. Treatment of latent infection to achieve tuberculosis elimination in low-incidence countries. PLoS Med. 2019; 16(6):e1002824. PMID: 31170161.28. Noh CS, Kim HI, Choi H, Kim Y, Kim CH, Choi JH, et al. Completion rate of latent tuberculosis infection treatment in patients aged 65 years and older. Respir Med. 2019; 157:52–58. PMID: 31522030.29. Chan PC, Lee PH, Lu MJ, Huang YC, Feng TY, Chen WW, et al. Tolerability of rifapentine-based regimens in latent tuberculosis infection treatment in the elderly. Eur Respir J. 2019; 53(3):1802396. PMID: 30886023.30. Feng JY, Huang WC, Lin SM, Wang TY, Lee SS, Shu CC, et al. Safety and treatment completion of latent tuberculosis infection treatment in the elderly population—A prospective observational study in Taiwan. Int J Infect Dis. 2020; 96:550–557. PMID: 32434083.31. Lee H, Koo GW, Min JH, Park TS, Park DW, Moon JY, et al. Factors associated with non-initiation of latent tuberculosis treatment among healthcare workers with a positive interferon-gamma releasing assay. Sci Rep. 2019; 9(1):61. PMID: 30635600.32. Mirtskhulava V, Whitaker JA, Kipiani M, Harris DA, Tabagari N, Owen-Smith AA, et al. Determinants of tuberculosis infection control-related behaviors among healthcare workers in the country of Georgia. Infect Control Hosp Epidemiol. 2015; 36(5):522–528. PMID: 25648218.33. Dobler CC, Bosnic-Anticevich S, Armour CL. Physicians’ perspectives on communication and decision making in clinical encounters for treatment of latent tuberculosis infection. ERJ Open Res. 2018; 4(1):00146-2017. PMID: 29577042.34. Kim HW, Kim JS. One step toward a low tuberculosis-burden country: screening for tuberculosis infection among the immigrants and refugees. Tuberc Respir Dis. 2020; 83(1):104–105.35. Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med. 2017; 167(4):248–255. PMID: 28761946.36. Yuen CM, Seddon JA, Keshavjee S, Dodd PJ. Risk-benefit analysis of tuberculosis infection testing for household contact management in high-burden countries: a mathematical modelling study. Lancet Glob Health. 2020; 8(5):e672–e680. PMID: 32353315.37. Pease C, Zwerling A, Mallick R, Patterson M, Demaio P, Finn S, et al. The latent tuberculosis infection cascade of care in Iqaluit, Nunavut, 2012-2016. BMC Infect Dis. 2019; 19(1):890. PMID: 31651260.38. Park SH, Lee SJ, Cho YJ, Jeong YY, Kim HC, Lee JD, et al. A prospective cohort study of latent tuberculosis in adult close contacts of active pulmonary tuberculosis patients in Korea. Korean J Intern Med. 2016; 31(3):517–524. PMID: 27052266.39. Rustage K, Lobe J, Hayward SE, Kristensen KL, Margineanu I, Stienstra Y, et al. Initiation and completion of treatment for latent tuberculosis infection in migrants globally: a systematic review and meta-analysis. Lancet Infect Dis. 2021; 21(12):1701–1712. PMID: 34363771.40. Barss L, Moayedi-Nia S, Campbell JR, Oxlade O, Menzies D. Interventions to reduce losses in the cascade of care for latent tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2020; 24(1):100–109. PMID: 32005312.41. Alsdurf H, Dick M. Identifying gaps in the quality of latent tuberculosis infection care. J Clin Tuberc Other Mycobact Dis. 2020; 18:100142. PMID: 31956699.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of Latent Tuberculosis Infection in Healthcare Workers
- Diagnosis and Treatment of Latent Tuberculosis Infection for Healthcare Workers
- Analysis of Prevalence and Risk Factors for Latent Tuberculosis Infection among Healthcare Workers
- The Prevalence and Risk Factors of Latent Tuberculosis Infection among Health Care Workers Working in a Tertiary Hospital in South Korea
- Diagnosis and Treatment of Latent Tuberculosis Infection: The Updated 2017 Korean Guidelines