Intest Res.
2022 Apr;20(2):203-212. 10.5217/ir.2021.00020.
Fecal S100A12 is associated with future hospitalization and step-up of medical treatment in patients with Crohn’s disease in clinical remission: a pilot study
- Affiliations
-
- 1Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Division of Gastroenterology, Mount Sinai Hospital, University of Toronto, Toronto, ON, Canada
- 3Inflammatory Bowel Disease Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2529566
- DOI: http://doi.org/10.5217/ir.2021.00020
Abstract
- Background/Aims
Fecal S100A12 (FS) and serum S100A12 (SS) have been reported as novel biomarkers that accurately reflect intestinal inflammation. We evaluated if FS and SS in comparison to fecal calprotectin (FC) are associated with poor future outcomes in clinically quiescent Crohn’s disease (CD) patients.
Methods
We prospectively enrolled 49 CD patients in clinical remission (Crohn’s Disease Activity Index [CDAI] < 150 for the past 6 months). Patients were followed for a median period of 4.4 years (interquartile range [IQR], 4.3–4.5). The following outcomes were evaluated: clinical relapse, CD-related hospitalization, step-up of medical treatment, and CD-related intestinal resection. Cox proportional-hazard regression model was constructed to assess the association of baseline markers with time-to-event outcomes.
Results
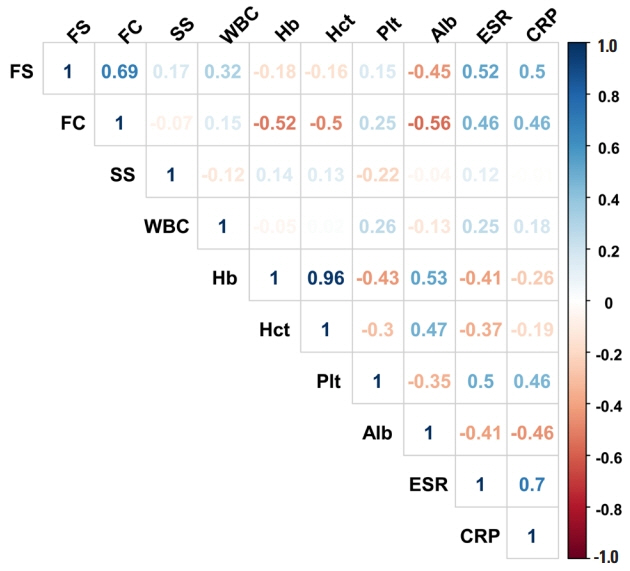
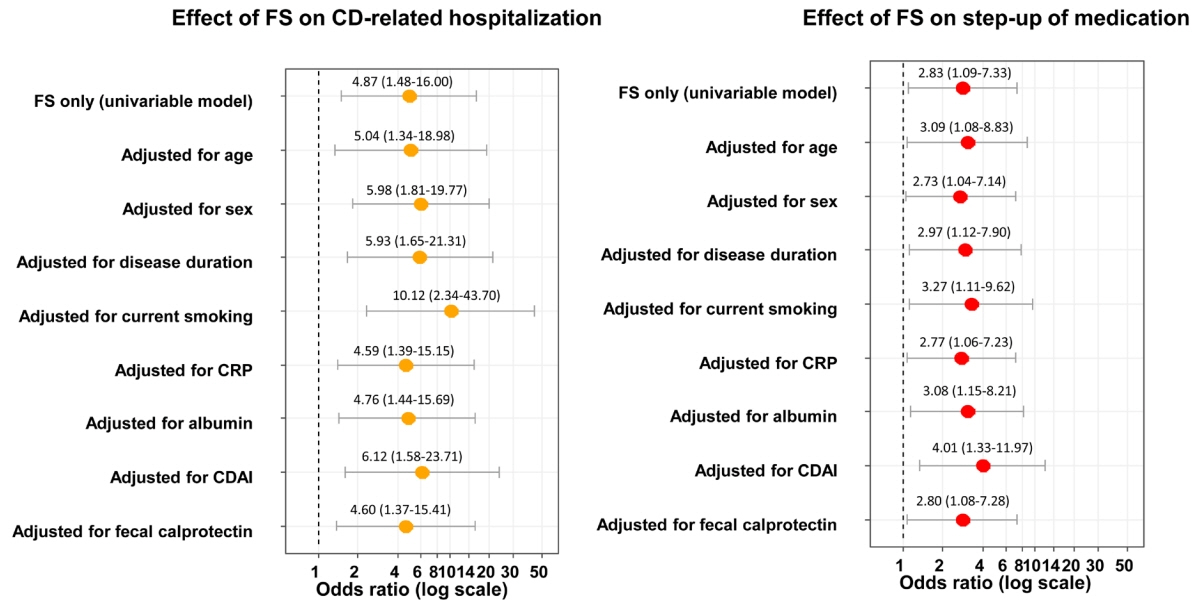
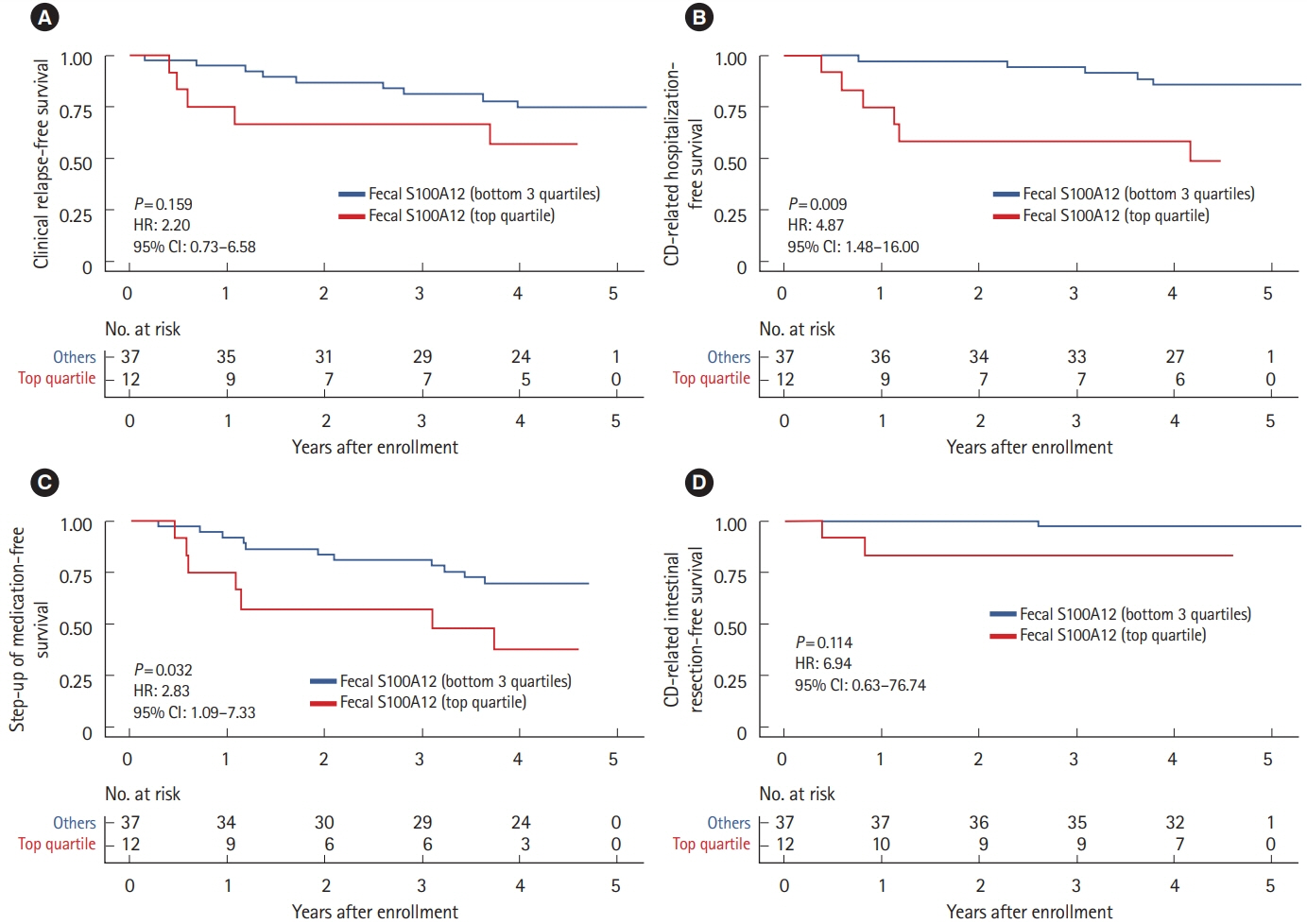
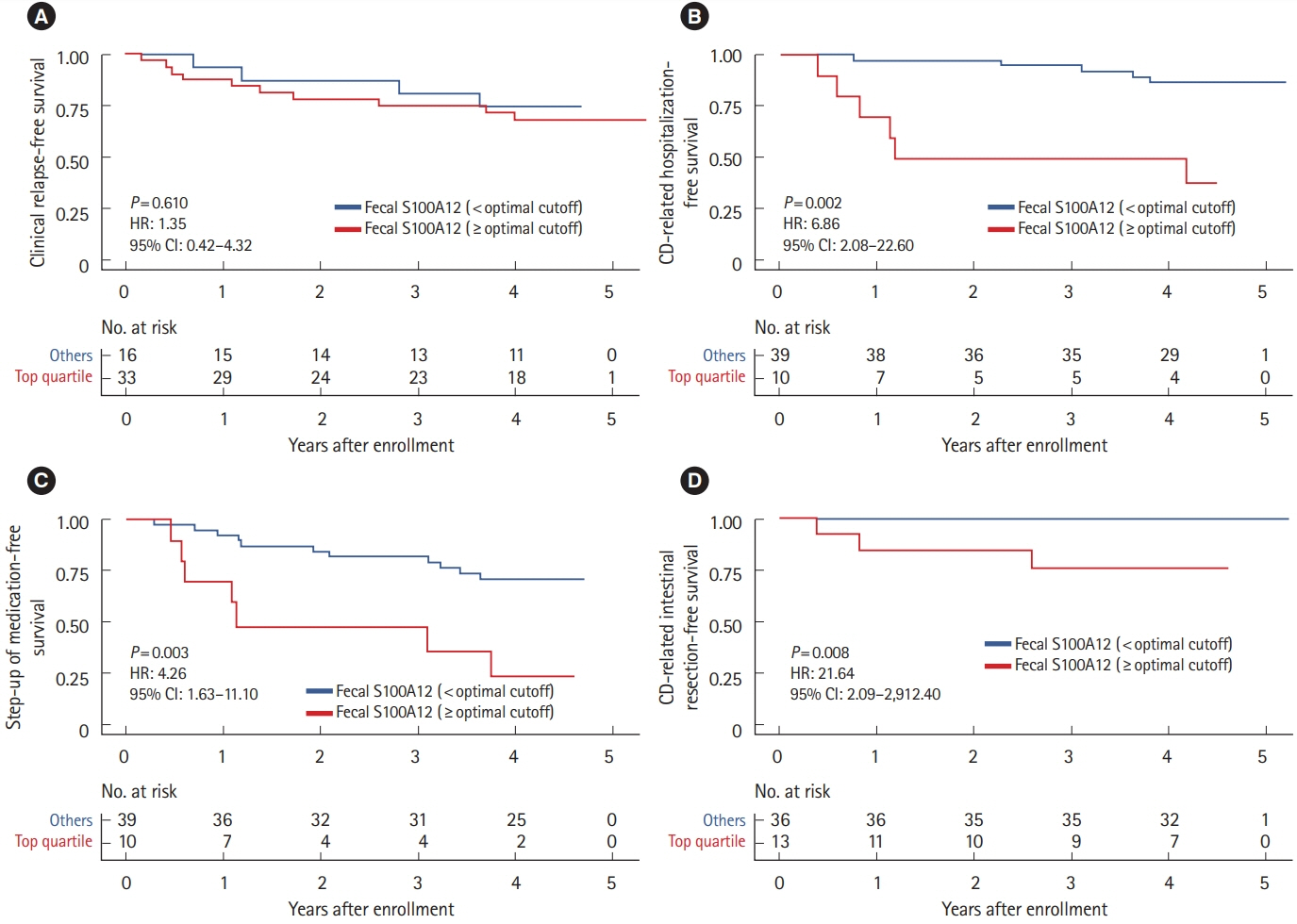
The median levels of baseline FS, FC, and SS were 0.042 mg/kg (IQR, 0.005–0.179), 486.8 mg/kg (IQR, 203.5–886.8) and 1,398.2 ng/mL (IQR, 791.8–2,759.9), respectively. FS correlated with FC (r = 0.689), erythrocyte sedimentation rate (r = 0.524), C-reactive protein (r = 0.499), and albumin (r = –0.446), but not with CDAI (r = 0.045). Interestingly, increased FS (top quartile) was associated with a 4.9-fold increased rate of future CD-related hospitalization (P= 0.009) and a 2.8-fold increased rate of step-up of medical treatment (P= 0.032), whereas increased FC and SS were not. These findings remained significant after adjusting for age, sex, disease duration, current smoking, C-reactive protein, serum albumin, CDAI, and FC, individually.
Conclusions
In this pilot study, increased FS and not FC or SS, was significantly associated with increased rates of future CD-related hospitalization and step-up of medical treatment among CD patients in clinical remission.
Keyword
Figure
Reference
-
1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017; 389:1741–1755.
Article2. Watanabe K. Clinical management for small bowel of Crohn’s disease in the treat-to-target era: now is the time to optimize treatment based on the dominant lesion. Intest Res. 2020; 18:347–354.
Article3. Røseth AG, Fagerhol MK, Aadland E, Schjønsby H. Assessment of the neutrophil dominating protein calprotectin in feces: a methodologic study. Scand J Gastroenterol. 1992; 27:793–798.
Article4. Foell D, Wittkowski H, Ren Z, et al. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008; 216:183–192.
Article5. Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015; 110:802–819.
Article6. Kaibullayeva J, Ualiyeva A, Oshibayeva A, Dushpanova A, Marshall JK. Prevalence and patient awareness of inflammatory bowel disease in Kazakhstan: a cross-sectional study. Intest Res. 2020; 18:430–437.
Article7. Rokkas T, Portincasa P, Koutroubakis IE. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: a diagnostic accuracy meta-analysis. J Gastrointestin Liver Dis. 2018; 27:299–306.
Article8. Simon EG, Wardle R, Thi AA, Eldridge J, Samuel S, Moran GW. Does fecal calprotectin equally and accurately measure disease activity in small bowel and large bowel Crohn’s disease? A systematic review. Intest Res. 2019; 17:160–170.
Article9. Tibble JA, Sigthorsson G, Bridger S, Fagerhol MK, Bjarnason I. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000; 119:15–22.
Article10. Gisbert JP, Bermejo F, Pérez-Calle JL, et al. Fecal calprotectin and lactoferrin for the prediction of inflammatory bowel disease relapse. Inflamm Bowel Dis. 2009; 15:1190–1198.
Article11. D’Incà R, Dal Pont E, Di Leo V, et al. Can calprotectin predict relapse risk in inflammatory bowel disease? Am J Gastroenterol. 2008; 103:2007–2014.
Article12. Costa F, Mumolo MG, Ceccarelli L, et al. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005; 54:364–368.
Article13. Musci JO, Cornish JS, Däbritz J. Utility of surrogate markers for the prediction of relapses in inflammatory bowel diseases. J Gastroenterol. 2016; 51:531–547.
Article14. Kaiser T, Langhorst J, Wittkowski H, et al. Faecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007; 56:1706–1713.
Article15. Sidler MA, Leach ST, Day AS. Fecal S100A12 and fecal calprotectin as noninvasive markers for inflammatory bowel disease in children. Inflamm Bowel Dis. 2008; 14:359–366.
Article16. Däbritz J, Langhorst J, Lügering A, et al. Improving relapse prediction in inflammatory bowel disease by neutrophil-derived S100A12. Inflamm Bowel Dis. 2013; 19:1130–1138.
Article17. Boschetti G, Garnero P, Moussata D, et al. Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn’s disease. Inflamm Bowel Dis. 2015; 21:331–336.
Article18. Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170:2–6.
Article19. Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976; 70:439–444.20. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–753.
Article21. Reenaers C, Bossuyt P, Hindryckx P, Vanpoucke H, Cremer A, Baert F. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United European Gastroenterol J. 2018; 6:1117–1125.
Article22. Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007; 81:28–37.
Article23. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017; 390:2779–2789.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Pro-inflammatory Protein S100A12 (EN-RAGE) in Behcet's Disease and Its Association with Disease Activity: A Pilot Study
- Fecal Calprotectin Assay at an Early Stage of Treatment Can Be Used as a Surrogate Marker to Predict Clinical Remission and Mucosal Healing in Pediatric Crohn’s Disease
- Surgical treatment of perianal fistula in Crohn's disease
- Primary surgery versus pharmacotherapy for newly diagnosed ileocecal Crohn’s disease: a hospital-based cohort study
- Fecal microbiota transplantation for refractory Crohn's disease