J Korean Med Sci.
2022 Apr;37(16):e128. 10.3346/jkms.2022.37.e128.
Analysis of Adverse Drug Reactions to First-Line Anti-Tuberculosis Drugs Using the Korea Adverse Event Reporting System
- Affiliations
-
- 1Department of Pulmonology and Allergy, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- 2Allergy and Clinical Immunology Research Center, Hallym University College of Medicine, Chuncheon, Korea
- 3Department of Pathology, Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Korea
- KMID: 2529295
- DOI: http://doi.org/10.3346/jkms.2022.37.e128
Abstract
- Background
Adverse drug reactions (ADRs) to first-line anti-tuberculosis (TB) drugs are common; however, there have been few reports of nationwide epidemiologic studies on ADRs to anti-TB drugs in Korea. This study aimed to investigate the clinical characteristics of various ADRs to first-line anti-TB drugs using a nationwide database of ADRs.
Methods
We used the Korea Adverse Event Reporting System (KAERS) database (2009– 2018). The study subjects were selected using the Korean Standard Classification of Diseases codes for pulmonary and extrapulmonary TB and electronic data interchange codes for isoniazid (INH), rifampicin (RIF), ethambutol (ETB), and pyrazinamide (PZA). The causality assessment of “possible,” “probable,” or “certain” by World Health Organization-Uppsala Monitoring Center System causality category was selected.
Results
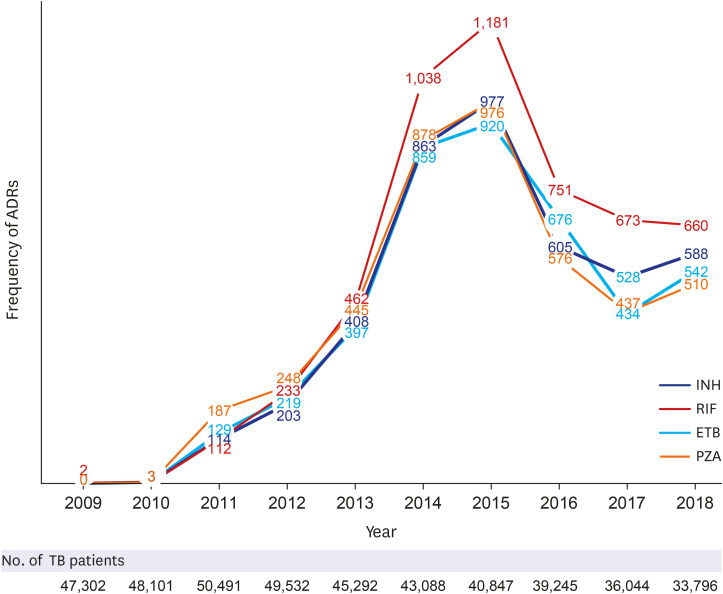
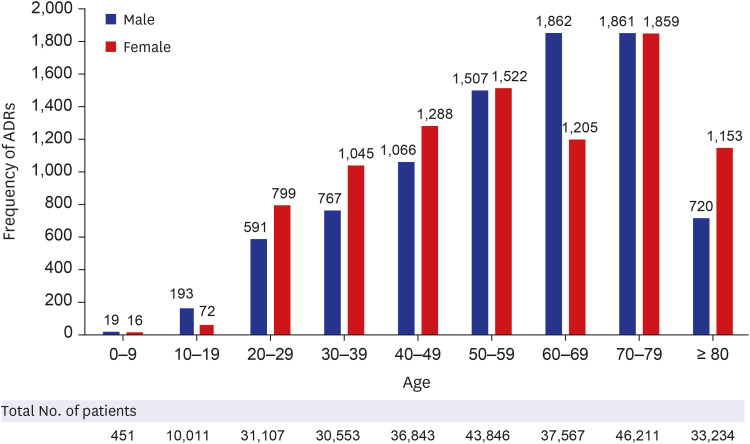
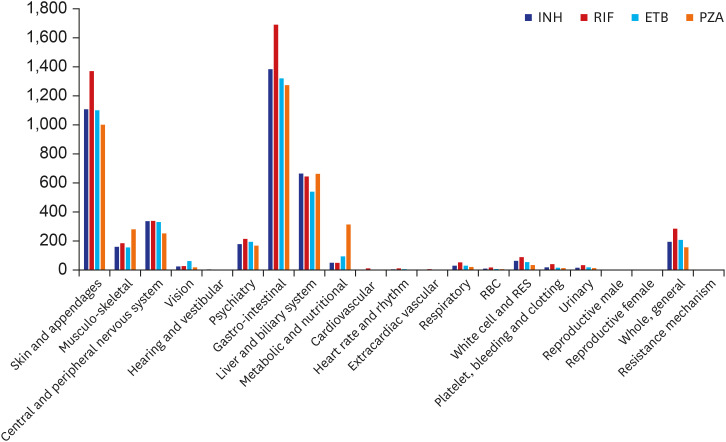
A total of 1,562,024 ADRs were reported in the KIDS-KAERS database from 2009 to 2018, where ADRs to first-line anti-TB drugs were 17,843 cases (1.14%). The most common causative drugs were RIF (28.7%), INH (24.0%), ETB (23.4%), and PZA (23.9%) in that order. 48.5% of cases were reported in the older patients (≥ 60 years). According to organ system, gastro-intestinal system disorder was most common (32.0%), followed by skin and appendage (25.9%), liver and biliary system (14.2%). Nausea was the most common ADR (14.6%), followed by hepatic enzyme elevation (14.2%), rash (11.7%), pruritus (9.1%), vomiting (8.9%), and urticaria (4.2%). Most ADRs appeared within 1 month, but ADRs such as neuropathy, paresthesia, hematologic abnormalities, renal function abnormalities and liver enzyme abnormality were also often reported after 2 months.
Conclusion
Our data are clinically informative for recognizing and coping with ADRs of antiTB drugs.
Figure
Reference
-
1. Global tuberculosis report 2020. Updated 2020. Accessed March 1, 2021. https://www.who.int/publications/i/item/9789240013131 .2. Gholami K, Kamali E, Hajiabdolbaghi M, Shalviri G. Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patients. Pharm Pract (Granada). 2006; 4(3):134–138. PMID: 25214900.3. Singh A, Prasad R, Balasubramanian V, Gupta N, Gupta P. Prevalence of adverse drug reaction with first-line drugs among patients treated for pulmonary tuberculosis. Clin Epidemiol Glob health. 2015; 3:S80–S90.4. Kim SH, Lee BH, Lee KD, Park JS, Kim YS, Jee YK, et al. The prevalence of adverse drug reactions to a short course anti-tuberculosis regimen. Korean J Med. 2007; 73(5):496–502.5. Carroll MW, Lee M, Cai Y, Hallahan CW, Shaw PA, Min JH, et al. Frequency of adverse reactions to first- and second-line anti-tuberculosis chemotherapy in a Korean cohort. Int J Tuberc Lung Dis. 2012; 16(7):961–966. PMID: 22584241.6. Choi NK, Park BJ. Adverse drug reaction surveillance system in Korea. J Prev Med Public Health. 2007; 40(4):278–284. PMID: 17693730.7. Statistics on reported ICSRs. Updated 2021. Accessed March 1, 2021. https://www.drugsafe.or.kr/iwt/ds/en/report/EgovICSRStatistics.do .8. Regal B. Finally a pharmacovigilant India. Uppsala Rep. 2004; 25(6):7–8.9. Shalviri G, Mohammad K, Majdzadeh R, Gholami K. Applying quantitative methods for detecting new drug safety signals in pharmacovigilance national database. Pharmacoepidemiol Drug Saf. 2007; 16(10):1136–1140. PMID: 17705214.10. Meyboom R, Royer R. Causality classification at pharmacovigilance centres in the European Community. Pharmacoepidemiol Drug Saf. 1992; 1(2):87–97.11. Nahler G. Dictionary of Pharmaceutical Medicine. Wien, Austria: SpringerWienNewYork;2009. p. 192–193.12. WHO-ART legacy service. Updated 2019. Accessed May 10, 2019. https://www.who-umc.org/vigibase/services/learn-more-about-who-art/ .13. Annual report on the notified tuberculosis in Korea, 2020. Updated 2020. Accessed March 1, 2021. https://tbzero.kdca.go.kr/tbzero/contents.do .14. Dhingra VK, Rajpal S, Aggarwal N, Aggarwaln JK, Shadab K, Jain SK. Adverse drug reactions observed during DOTS. J Commun Dis. 2004; 36(4):251–259. PMID: 16506547.15. Lv X, Tang S, Xia Y, Wang X, Yuan Y, Hu D, et al. Adverse reactions due to directly observed treatment strategy therapy in Chinese tuberculosis patients: a prospective study. PLoS One. 2013; 8(6):e65037. PMID: 23750225.16. Farazi A, Sofian M, Jabbariasl M, Keshavarz S. Adverse reactions to antituberculosis drugs in Iranian tuberculosis patients. Tuberc Res Treat. 2014; 2014:412893. PMID: 25506427.17. Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003; 167(11):1472–1477. PMID: 12569078.18. Maciel EL, Guidoni LM, Favero JL, Hadad DJ, Molino LP, Jonhson JL, et al. Adverse effects of the new tuberculosis treatment regimen recommended by the Brazilian Ministry of Health. J Bras Pneumol. 2010; 36(2):232–238. PMID: 20485945.19. Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996; 9(10):2026–2030. PMID: 8902462.20. Marra F, Marra CA, Bruchet N, Richardson K, Moadebi S, Elwood RK, et al. Adverse drug reactions associated with first-line anti-tuberculosis drug regimens. Int J Tuberc Lung Dis. 2007; 11(8):868–875. PMID: 17705952.21. Chung-Delgado K, Revilla-Montag A, Guillen-Bravo S, Velez-Segovia E, Soria-Montoya A, Nuñez-Garbin A, et al. Factors associated with anti-tuberculosis medication adverse effects: a case-control study in Lima, Peru. PLoS One. 2011; 6(11):e27610. PMID: 22110689.22. Jin HJ, Kang DY, Nam YH, Ye YM, Koh YI, Hur GY, et al. Severe cutaneous adverse reactions to anti-tuberculosis drugs in Korean patients. Allergy Asthma Immunol Res. 2021; 13(2):245–255. PMID: 33474859.23. Ban GY, Jeong YJ, Lee SH, Shin SS, Shin YS, Park HS, et al. Efficacy and tolerability of desensitization in the treatment of delayed drug hypersensitivities to anti-tuberculosis medications. Respir Med. 2019; 147:44–50. PMID: 30704698.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Signal Detection of Adverse Event of Metoclopramide in Korea Adverse Event Reporting System (KAERS)
- Analysis of Important Medical Adverse Events and Signals Related with Cyclosporine and Tacrolimus Using the FDA Adverse Event Reporting System (FAERS) Database
- Signal Detection for Adverse Events of Finasteride Using Korea Adverse Event Reporting System (KAERS) Database
- Relief System for Adverse Drug Reactions in Korea
- Recent Change of the Etiology of Drug Induced Anaphylaxis in a Korean Tertiary Care Hospital