J Yeungnam Med Sci.
2022 Apr;39(2):89-97. 10.12701/jyms.2021.01669.
Coronavirus disease 2019 (COVID-19) vaccine platforms: how novel platforms can prepare us for future pandemics: a narrative review
- Affiliations
-
- 1BK21 Graduate Program, Department of Biomedical Sciences, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2529266
- DOI: http://doi.org/10.12701/jyms.2021.01669
Abstract
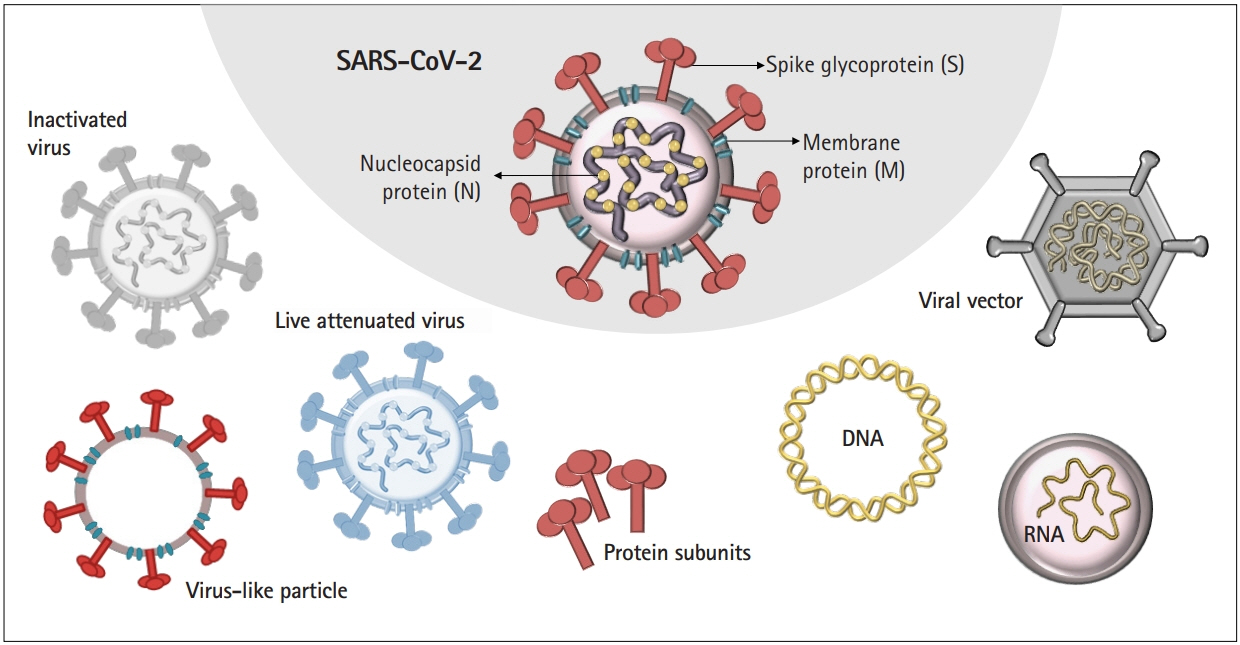
- More than 2 years after the explosion of the coronavirus disease 2019 (COVID-19) pandemic, extensive efforts have been made to develop safe and efficacious vaccines against infections with severe acute respiratory syndrome coronavirus 2. The pandemic has opened a new era of vaccine development based on next-generation platforms, including messenger RNA (mRNA)-based technologies, and paved the way for the future of mRNA-based therapeutics to provide protection against a wide range of infectious diseases. Multiple vaccines have been developed at an unprecedented pace to protect against COVID-19 worldwide. However, important knowledge gaps remain to be addressed, especially in terms of how vaccines induce immunogenicity and efficacy in those who are elderly. Here, we discuss the various vaccine platforms that have been utilized to combat COVID-19 and emphasize how these platforms can be a powerful tool to react quickly to future pandemics.
Figure
Reference
-
References
1. World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard [Internet]. Geneva: WHO;2021. [cited 2021 Dec 14]. https://covid19.who.int/.2. Stadler K, Masignani V, Eickmann M, Becker S, Abrignani S, Klenk HD, et al. SARS: beginning to understand a new virus. Nat Rev Microbiol. 2003; 1:209–18.3. Janeway Jr CA, Travers P, Walport M, Shlomchik MJ. The distribution and functions of immunoglobulin isotypes. In : Janeway Jr CA, Travers P, Walport M, Shlomchik MJ, editors. Immunobiology: the immune system in health and disease. 5th ed. New York: Garland Science;2001.4. World Health Organization (WHO). Tracking SARS-CoV-2 variants [Internet]. Geneva: WHO;2021. [cited 2021 Dec 14]. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.5. World Health Organization (WHO). Weekly epidemiological update on COVID-19-25 [Internet]. Geneva: WHO;2022. [cited 2022 Jan 17]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---25-january-2022.6. Centers for Disease Control and Prevention (CDC). COVID data tracker: variant proportions [Internet]. Atlanta: CDC;2022. [cited 2022 Jan 17]. https://covid.cdc.gov/covid-data-tracker/#monitoring-varaint-heading.7. Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, et al. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021; 4:e2140364.8. Bansal A, Trieu MC, Mohn KGI, Cox RJ. Safety, immunogenicity, efficacy and effectiveness of inactivated influenza vaccines in healthy pregnant women and children under 5 years: an evidence-based clinical review. Front Immunol. 2021; 12:744774.9. Pepin S, Dupuy M, Borja-Tabora CF, Montellano M, Bravo L, Santos J, et al. Efficacy, immunogenicity, and safety of a quadrivalent inactivated influenza vaccine in children aged 6-35 months: a multi-season randomised placebo-controlled trial in the Northern and Southern hemispheres. Vaccine. 2019; 37:1876–84.10. Ott JJ, Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis. 2013; 17:e939–44.11. Centers for Disease Control and Prevention (CDC). Polio vaccination [Internet]. Atlanta: CDC;2018. [cited 2022 Jan 17]. https://www.cdc.gov/vaccines/vpd/polio/index.html.12. Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021; 21:181–92.13. Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021; 398:213–22.14. Wang H, Zhang Y, Huang B, Deng W, Quan Y, Wang W, et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020; 182:713–21.15. Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021; 21:39–51.16. Guo W, Duan K, Zhang Y, Yuan Z, Zhang YB, Wang Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 1/2 trial. EClinicalMedicine. 2021; 38:101010.17. Li XN, Huang Y, Wang W, Jing QL, Zhang CH, Qin PZ, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case-control real-world study. Emerg Microbes Infect. 2021; 10:1751–9.18. Russell WC. Adenoviruses: update on structure and function. J Gen Virol. 2009; 90(Pt 1):1–20.19. Buller RE, Runnebaum IB, Karlan BY, Horowitz JA, Shahin M, Buekers T, et al. A phase I/II trial of rAd/p53 (SCH 58500) gene replacement in recurrent ovarian cancer. Cancer Gene Ther. 2002; 9:553–66.20. Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004; 10:616–29.21. Dolzhikova IV, Zubkova OV, Tukhvatulin AI, Dzharullaeva AS, Tukhvatulina NM, Shcheblyakov DV, et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: an open phase I/II trial in healthy adults in Russia. Hum Vaccin Immunother. 2017; 13:613–20.22. Astuti I. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020; 14:407–12.23. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020; 396:467–78.24. Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021; 397:881–91.25. Sadoff J, Le Gars M, Shukarev G, Heerwegh D, Truyers C, de Groot AM, et al. Interim results of a phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med. 2021; 384:1824–35.26. Bos R, Rutten L, van der Lubbe JE, Bakkers MJ, Hardenberg G, Wegmann F, et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020; 5:91.27. Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021; 397:671–81.28. Eichinger S, Warkentin TE, Greinacher A. Thrombotic thrombocytopenia after ChAdOx1 nCoV-19 vaccination: reply. N Engl J Med. 2021; 385:e11.29. Health Alert Network; Centers for Disease Control and Prevention (CDC). Emergency preparedness and response: cases of cerebral venous sinus thrombosis with thrombocytopenia after receipt of the Johnson & Johnson COVID-19 vaccine [Internet]. Atlanta: CDC;2021. [cited 2022 Jan 17]. https://emergency.cdc.gov/han/2021/han00442.asp.30. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383:2603–15.31. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020; 367:1260–3.32. Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021; 385:1761–73.33. Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature. 2021; 595:572–7.34. Ahmed SF, Quadeer AA, McKay MR. SARS-CoV-2 T cell responses elicited by COVID-19 vaccines or infection are expected to remain robust against Omicron. Viruses. 2022; 14:79.35. Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep Med. 2021; 2:100355.36. Stanojevic M, Geiger A, Ostermeier B, Sohai D, Lazarski C, Lang H, et al. Spike-directed vaccination elicits robust spike-specific T-cell response, including to mutant strains. Cytotherapy. 2022; 24:10–5.37. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021; 385:585–94.38. Groß R, Zanoni M, Seidel A, Conzelmann C, Gilg A, Krnavek D, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 2022; 75:103761.39. Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Alroy-Preis S, et al. Protection against COVID-19 by BNT162b2 booster across age groups. N Engl J Med. 2021; 385:2421–30.40. Perrie Y, Crofts F, Devitt A, Griffiths HR, Kastner E, Nadella V. Designing liposomal adjuvants for the next generation of vaccines. Adv Drug Deliv Rev. 2016; 99(Pt A):85–96.41. Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020; 383:2427–38.42. El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021; 385:1774–85.43. Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative effectiveness of moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions: United States, March-August 2021. MMWR Morb Mortal Wkly Rep. 2021; 70:1337–43.44. Castiello T, Georgiopoulos G, Finocchiaro G, Claudia M, Gianatti A, Delialis D, et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2022; 27:251–61.45. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021; 385:1078–90.46. Colella G, Orlandi M, Cirillo N. Bell’s palsy following COVID-19 vaccination. J Neurol. 2021; 268:3589–91.47. Repajic M, Lai XL, Xu P, Liu A. Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy. Brain Behav Immun Health. 2021; 13:100217.48. Ozonoff A, Nanishi E, Levy O. Bell’s palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021; 21:450–2.49. Teixeira FM, Teixeira HC, Ferreira AP, Rodrigues MF, Azevedo V, Macedo GC, et al. DNA vaccine using Mycobacterium bovis Ag85B antigen induces partial protection against experimental infection in BALB/c mice. Clin Vaccine Immunol. 2006; 13:930–5.50. Kodihalli S, Kobasa DL, Webster RG. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine. 2000; 18:2592–9.51. Rai N, Kaushik P, Rai A. Development of rabies DNA vaccine using a recombinant plasmid. Acta Virol. 2005; 49:207–10.52. Tebas P, Yang S, Boyer JD, Reuschel EL, Patel A, Christensen-Quick A, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine. 2021; 31:100689.53. Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A. 2017; 114:E7348–57.54. Magnusson SE, Altenburg AF, Bengtsson KL, Bosman F, de Vries RD, Rimmelzwaan GF, et al. Matrix-M™ adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol Res. 2018; 66:224–33.55. Reimer JM, Karlsson KH, Lövgren-Bengtsson K, Magnusson SE, Fuentes A, Stertman L. Matrix-M™ adjuvant induces local recruitment, activation and maturation of central immune cells in absence of antigen. PLoS One. 2012; 7:e41451.56. Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med. 2021; 385:1172–83.57. Arunachalam PS, Walls AC, Golden N, Atyeo C, Fischinger S, Li C, et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021; 594:253–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Review of COVID-19 Vaccines and Their Evidence in Older Adults
- Responses against infectious disease pandemics: a narrative review on COVID-19 A narrative review
- COVID-19 from the Perspective of a Gastroenterologist
- India’s efforts to achieve 1.5 billion COVID-19 vaccinations: a narrative review
- Steroid injections in pain management: influence on coronavirus disease 2019 vaccines