Cancer Res Treat.
2022 Apr;54(2):590-596. 10.4143/crt.2021.311.
Incidence Patterns and Outcomes of Ewing Sarcoma in South Korea (1999-2017): A Retrospective Analysis Using Korea Central Cancer Registry Data
- Affiliations
-
- 1Department of Pediatrics, Center for Pediatric Center, National Cancer Center, Goyang, Korea
- 2Division of Cancer Registration and Surveillance, National Cancer Center, Goyang, Korea
- 3Orthopaedic Oncology Clinic, Center for Rare Cancer, National Cancer Center, Goyang, Korea
- KMID: 2528228
- DOI: http://doi.org/10.4143/crt.2021.311
Abstract
- Purpose
Due to low incidence, epidemiologic data of Ewing sarcoma in the Asian population are scarce. We aimed to examine the incidence pattern and outcome of patients with Ewing sarcoma in the Republic of Korea.
Materials and Methods
Data of patients with Ewing sarcoma diagnosed between 1999 and 2017 were obtained from the Korea Central Cancer Registry (KCCR). Incidence, clinical characteristics, and survival rates were analyzed and compared between different age groups.
Results
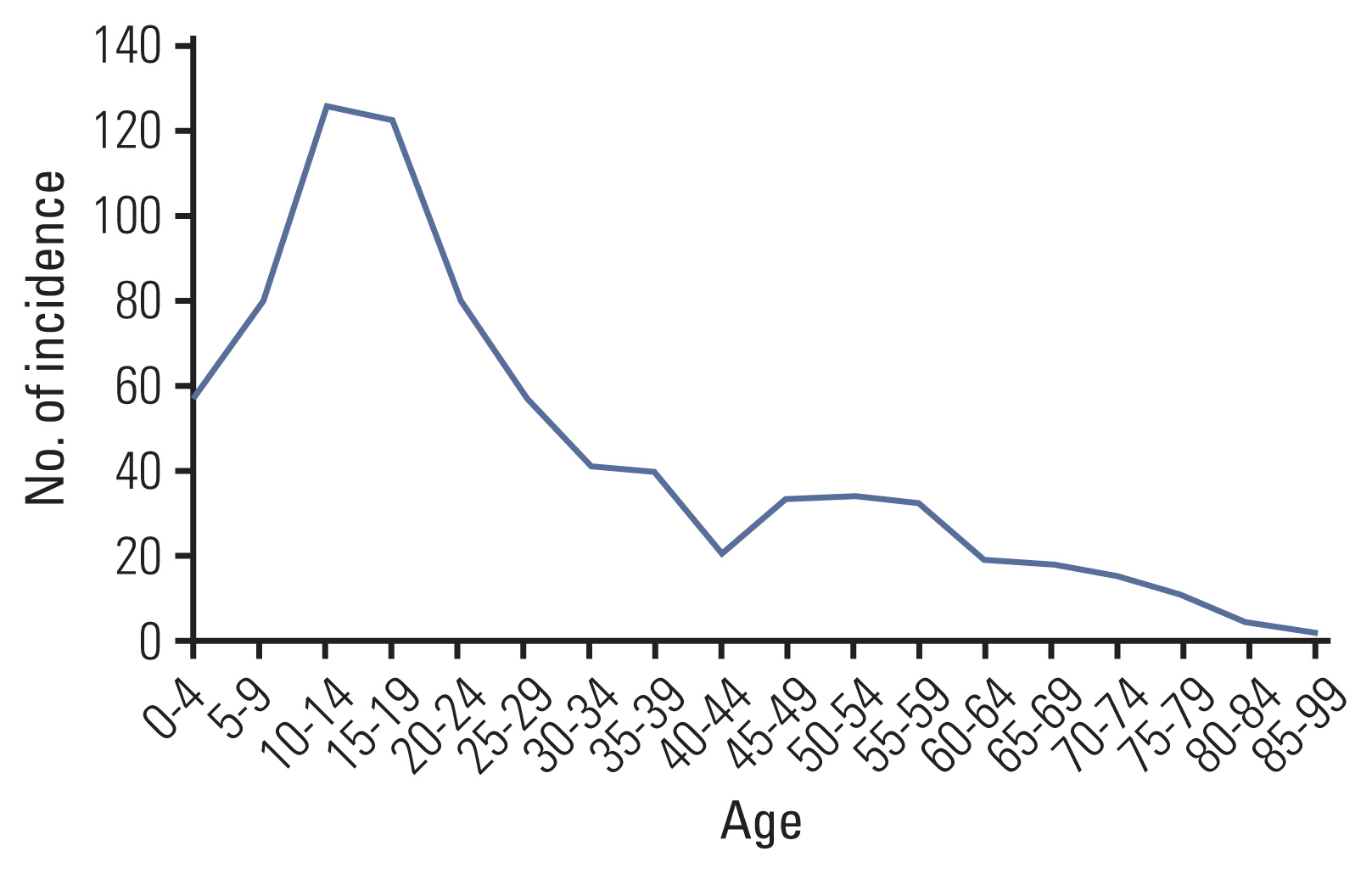
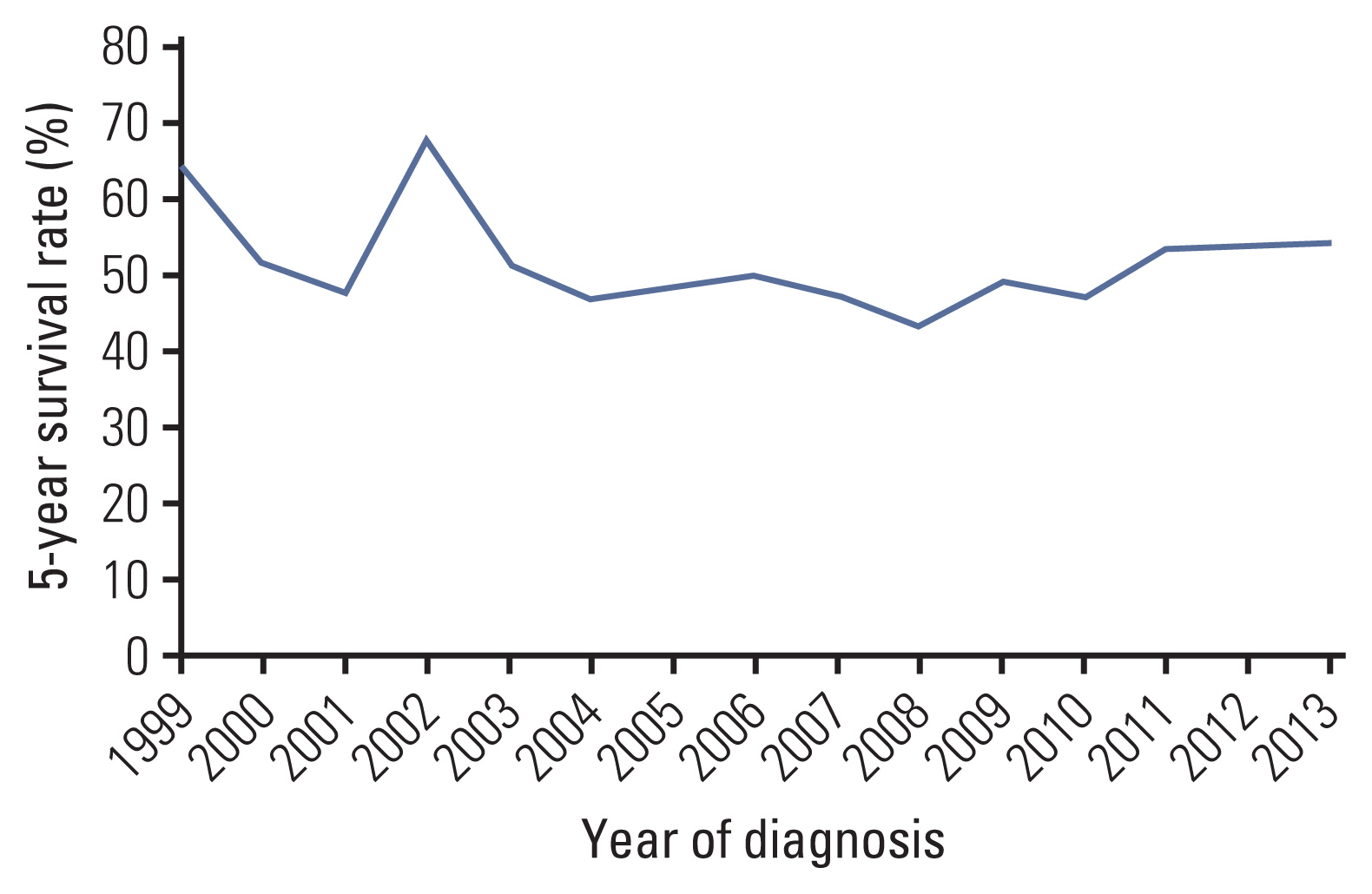
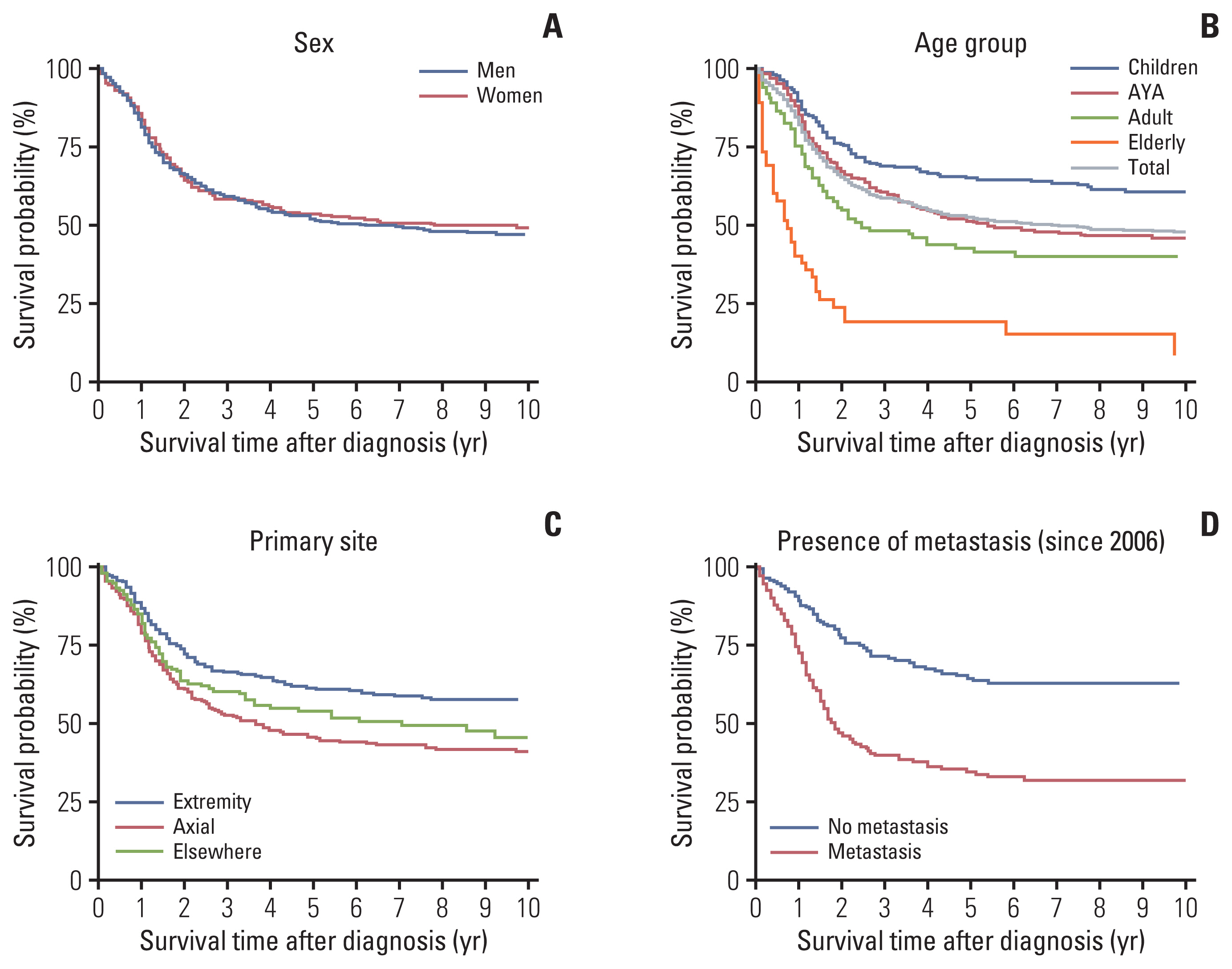
There were 788 cases (459 males, 329 females), with a median age at diagnosis of 20 years. The age-standardized rate of Ewing sarcoma was 1.01. The number of cases and incidence rates in each age group were as follows: children, 1.6; adolescents and young adults (AYA), 0.93; adults, 0.44; and elderly, 0.53. There were more male cases in children and the AYA group (p < 0.001). Extraskeletal tumors (p < 0.001), primary sites other than extremity (p=0.007), and presence of metastasis at diagnosis (p=0.031) were more frequent in the adults and elderly group. With a median survival time of 78 months, the 5-year overall survival (OS) rate of the entire cohort was 52%. Children fared best (5-year OS, 75%), and the 5-year OS of AYA patients (51%) approximated the OS of the entire cohort. A two-fold difference of 5-year OS was observed between adults and elderly patients (42% vs. 19%). On univariate and multivariate analyses, age ≥ 15 years and presence of metastasis were adverse prognostic factors.
Conclusion
This was the first epidemiologic study of Ewing sarcoma using the KCCR data. With a similar incidence to other Asian countries, the survival rate was slightly lower than that of Euro-American cases. Collaborative clinical studies are necessary to improve the outcome of Ewing sarcoma in low-incidence populations.
Keyword
Figure
Reference
-
References
1. Riggi N, Suva ML, Stamenkovic I. Ewing’s sarcoma. N Engl J Med. 2021; 384:154–64.
Article2. Jawad MU, Cheung MC, Min ES, Schneiderbauer MM, Koniaris LG, Scully SP. Ewing sarcoma demonstrates racial disparities in incidence-related and sex-related differences in outcome: an analysis of 1631 cases from the SEER database, 1973–2005. Cancer. 2009; 115:3526–36.
Article3. Worch J, Matthay KK, Neuhaus J, Goldsby R, DuBois SG. Ethnic and racial differences in patients with Ewing sarcoma. Cancer. 2010; 116:983–8.
Article4. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents. Lyon: IARC Press;2007.5. Anfinsen KP, Devesa SS, Bray F, Troisi R, Jonasdottir TJ, Bruland OS, et al. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976–2005). Cancer Epidemiol Biomarkers Prev. 2011; 20:1770–7.
Article6. Whelan J, McTiernan A, Cooper N, Wong YK, Francis M, Vernon S, et al. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int J Cancer. 2012; 131:E508–17.
Article7. Juergens C, Weston C, Lewis I, Whelan J, Paulussen M, Oberlin O, et al. Safety assessment of intensive induction with vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) in the treatment of Ewing tumors in the EURO-E.W.I.N.G. 99 clinical trial. Pediatr Blood Cancer. 2006; 47:22–9.
Article8. Miser JS, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Treatment of metastatic Ewing’s sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide: a Children’s Cancer Group and Pediatric Oncology Group study. J Clin Oncol. 2004; 22:2873–6.9. Perlman EJ, Dickman PS, Askin FB, Grier HE, Miser JS, Link MP. Ewing’s sarcoma: routine diagnostic utilization of MIC2 analysis: a Pediatric Oncology Group/Children’s Cancer Group Intergroup Study. Hum Pathol. 1994; 25:304–7.10. Zucker JM, Henry-Amar M. Therapeutic controlled trial in Ewing’s sarcoma. Report on the results of a trial by the Clinical Cooperative Group on Radio- and Chemotherapy of the E.O.R.T.C. Eur J Cancer. 1977; 13:1019–23.11. Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003; 348:694–701.
Article12. Korean Statistical Information Service [Internet]. Daejeon: Statisticak Korea;2021. [cited 2021 Feb 1]. Available from: https://kosis.kr/eng/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ETITLE&parmTabId=M_01_01 .13. Segi M. Cancer mortality for selected sites in 24 countries 1950–57. Sendai: Tohoku University School of Medicine;1960.14. Young JL, Roffers SD, Ries LA, Fritz AG, Hurlbut AA. SEER summary staging manual, 2000 coded and coding instructions. Bethesda, MD: National Cancer Institute;2001.15. Esteve J, Benhamou E, Raymond L. Statistical methods in cancer research. Vol. IV Descriptive epidemiology. Oxford: Oxford University Press;1994.16. Cox DR. Regression models and life tables. J R Stat Soc Series B. 1972; 34:187–220.17. Hung GY, Horng JL, Yen HJ, Yen CC, Chen WM, Chen PC, et al. Incidence patterns of primary bone cancer in taiwan (2003–2010): a population-based study. Ann Surg Oncol. 2014; 21:2490–8.
Article18. Keegan TH, Ries LA, Barr RD, Geiger AM, Dahlke DV, Pollock BH, et al. Comparison of cancer survival trends in the United States of adolescents and young adults with those in children and older adults. Cancer. 2016; 122:1009–16.
Article19. Fukushima T, Ogura K, Akiyama T, Takeshita K, Kawai A. Descriptive epidemiology and outcomes of bone sarcomas in adolescent and young adult patients in Japan. BMC Musculoskelet Disord. 2018; 19:297.
Article20. Arvand A, Denny CT. Biology of EWS/ETS fusions in Ewing’s family tumors. Oncogene. 2001; 20:5747–54.
Article21. Burchill SA. Ewing’s sarcoma: diagnostic, prognostic, and therapeutic implications of molecular abnormalities. J Clin Pathol. 2003; 56:96–102.
Article22. Kojima T, Asami S, Chin M, Yoshida Y, Mugishima H, Suzuki T. Detection of chimeric genes in Ewing’s sarcoma and its clinical applications. Biol Pharm Bull. 2002; 25:991–4.
Article23. Lee JA, Kim DH, Cho J, Lim JS, Koh JS, Yoo JY, et al. Treatment outcome of Korean patients with localized Ewing sarcoma family of tumors: a single institution experience. Jpn J Clin Oncol. 2011; 41:776–82.
Article24. Cotterill SJ, Ahrens S, Paulussen M, Jurgens HF, Voute PA, Gadner H, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000; 18:3108–14.
Article25. Fizazi K, Dohollou N, Blay JY, Guerin S, Le Cesne A, Andre F, et al. Ewing’s family of tumors in adults: multivariate analysis of survival and long-term results of multimodality therapy in 182 patients. J Clin Oncol. 1998; 16:3736–43.
Article26. Bacci G, Ferrari S, Comandone A, Zanone A, Ruggieri P, Longhi A, et al. Neoadjuvant chemotherapy for Ewing’s sarcoma of bone in patients older than thirty-nine years. Acta Oncol. 2000; 39:111–6.27. Martin RC 2nd, Brennan MF. Adult soft tissue Ewing sarcoma or primitive neuroectodermal tumors: predictors of survival? Arch Surg. 2003; 138:281–5.28. Lee J, Hoang BH, Ziogas A, Zell JA. Analysis of prognostic factors in Ewing sarcoma using a population-based cancer registry. Cancer. 2010; 116:1964–73.
Article