Blood Res.
2022 Mar;57(1):20-28. 10.5045/br.2022.2022043.
Management of immune thrombocytopenia: 2022 update of Korean experts recommendations
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea
- 2Department of Internal Medicine, Uijeongbu Eulji Medical Center, Eulji Univerisity, Seoul, Korea
- 3Department of Pediatrics, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Pediatrics, Ajou University School of Medicine, Ajou Univeristy Hospital, Suwon, Korea
- 5Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 6Division of Hematology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, Seoul, Korea
- 7Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Korea
- 8Department of Hematology-Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 9Department of Hematology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 10Department of Pediatrics, Chonnam National University Hwasun Hospital, Hwasun, Korea
- KMID: 2527493
- DOI: http://doi.org/10.5045/br.2022.2022043
Abstract
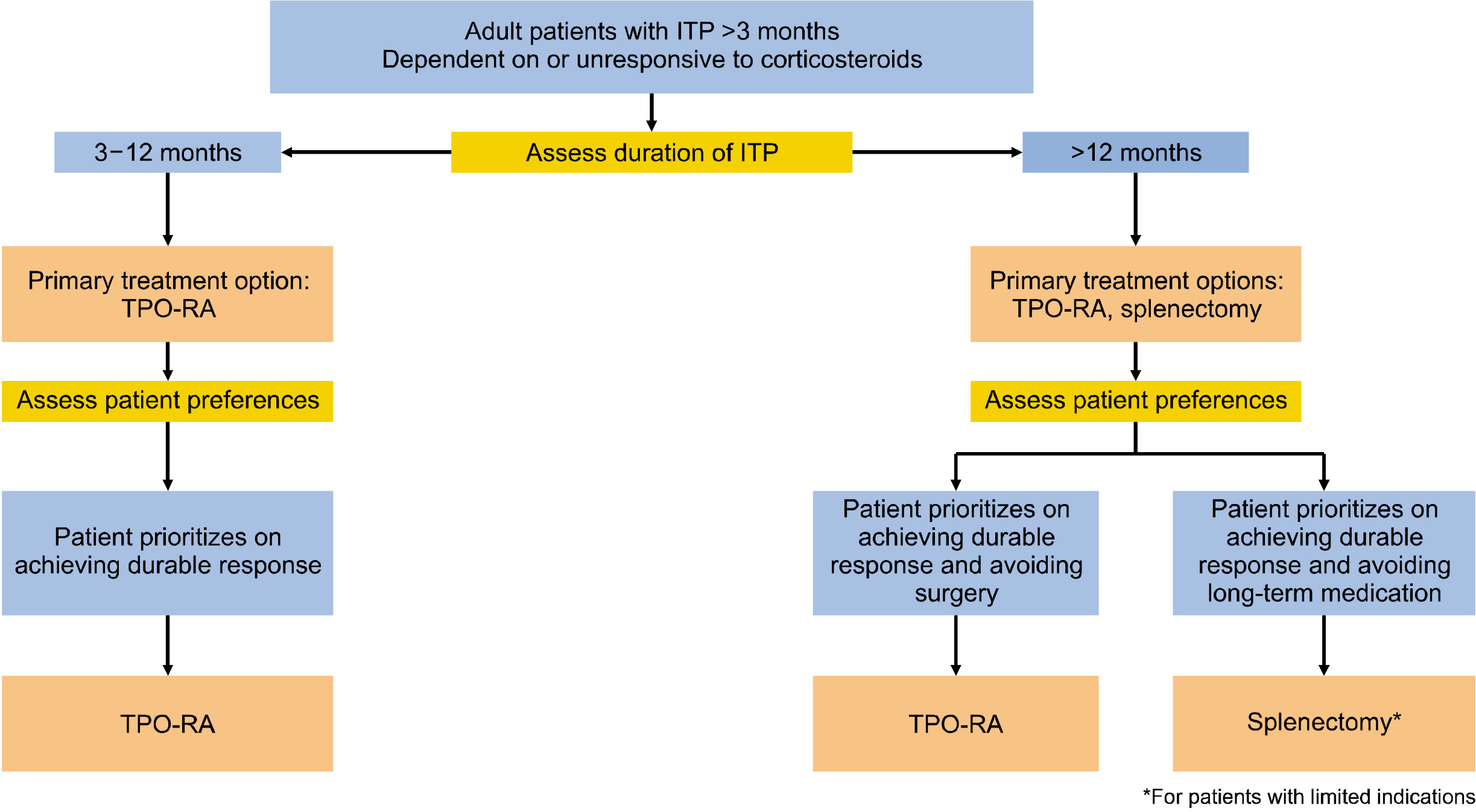
- Despite the availability of therapies to treat patients with immune thrombocytopenia (ITP), there is currently little data from randomized trials to assist clinicians in managing patients. The evidence-based guidelines of the Korean Society of Hematology Aplastic Anemia Working Party (KSHAAWP) are intended to support patients and physicians in the management of ITP. Experts from the KSHAAWP discussed and described this guideline according to the current treatment situation for ITP in Korea and finalized the guidelines. The expert panel recommended the management of ITP in adult and pediatric patients with newly diagnosed, persistent, and chronic disease refractory to first-line therapy with minor bleeding. Management approaches include observation and administration of corticosteroids, intravenous immunoglobulin, anti-D immunoglobulin, and thrombopoietin receptor agonists. Currently, evidence supporting strong recommendations for various management approaches is lacking. Therefore, a large focus was placed on shared decision-making, especially regarding second-line treatment.
Keyword
Figure
Cited by 1 articles
-
Recent advances in treatments of adult immune thrombocytopenia
Dae Sik Kim
Blood Res. 2022;57(S1):112-119. doi: 10.5045/br.2022.2022038.
Reference
-
1. Abrahamson PE, Hall SA, Feudjo-Tepie M, Mitrani-Gold FS, Logie J. 2009; The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur J Haematol. 83:83–9. DOI: 10.1111/j.1600-0609.2009.01247.x. PMID: 19245532.
Article2. Schoonen WM, Kucera G, Coalson J, et al. 2009; Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br J Haematol. 145:235–44. DOI: 10.1111/j.1365-2141.2009.07615.x. PMID: 19245432.
Article3. Segal JB, Powe NR. 2006; Prevalence of immune thrombocytopenia: analyses of administrative data. J Thromb Haemost. 4:2377–83. DOI: 10.1111/j.1538-7836.2006.02147.x. PMID: 16869934.
Article4. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. 2010; The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. Am J Hematol. 85:174–80. DOI: 10.1002/ajh.21616. PMID: 20131303.
Article5. Yong M, Schoonen WM, Li L, et al. 2010; Epidemiology of paediatric immune thrombocytopenia in the General Practice Research Database. Br J Haematol. 149:855–64. DOI: 10.1111/j.1365-2141.2010.08176.x. PMID: 20377590.
Article6. Lee JY, Lee JH, Lee H, et al. 2017; Epidemiology and management of primary immune thrombocytopenia: a nationwide population-based study in Korea. Thromb Res. 155:86–91. DOI: 10.1016/j.thromres.2017.05.010. PMID: 28525829.
Article7. Lim JH, Kim YK, Min SH, Kim SW, Lee YH, Lee JM. 2021; Epidemiology and viral etiology of pediatric immune thrombocytopenia through Korean public health data analysis. J Clin Med. 10:1356. DOI: 10.3390/jcm10071356. PMID: 33806145. PMCID: PMC8037772. PMID: 2161b583ca1c42dcb342194d51cfa654.
Article8. Park SH, Kwak SG, Kim JY. 2021; Incidence and prevalence of immune thrombocytopenia under the copayment waiver policy for pediatric patients in Korea: data from the National Health Claims Database. Lupus. 30:655–60. DOI: 10.1177/0961203321995247. PMID: 33593162.
Article9. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. 2009; The ITP syndrome: pathogenic and clinical diversity. Blood. 113:6511–21. DOI: 10.1182/blood-2009-01-129155. PMID: 19395674. PMCID: PMC2710913.
Article10. Neunert CE, Buchanan GR, Blanchette V, et al. 2009; Relationships among bleeding severity, health-related quality of life, and platelet count in children with immune thrombocytopenic purpura. Pediatr Blood Cancer. 53:652–4. DOI: 10.1002/pbc.21978. PMID: 19492316.
Article11. Neunert C, Noroozi N, Norman G, et al. 2015; Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J Thromb Haemost. 13:457–64. DOI: 10.1111/jth.12813. PMID: 25495497. PMCID: PMC4991942.
Article12. Neunert CE, Buchanan GR, Imbach P, et al. 2008; Severe hemorrhage in children with newly diagnosed immune thrombocytopenic purpura. Blood. 112:4003–8. DOI: 10.1182/blood-2008-03-138487. PMID: 18698007. PMCID: PMC2581983.
Article13. Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB. 2009; Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood. 114:4777–83. DOI: 10.1182/blood-2009-04-215525. PMID: 19767509. PMCID: PMC2786288.
Article14. Frederiksen H, Maegbaek ML, Nørgaard M. 2014; Twenty-year mortality of adult patients with primary immune thrombo-cytopenia: a Danish population-based cohort study. Br J Haematol. 166:260–7. DOI: 10.1111/bjh.12869. PMID: 24690142.
Article15. Kuter DJ, Mathias SD, Rummel M, et al. 2012; Health-related quality of life in nonsplenectomized immune thrombocytopenia patients receiving romiplostim or medical standard of care. Am J Hematol. 87:558–61. DOI: 10.1002/ajh.23163. PMID: 22460421.
Article16. Snyder CF, Mathias SD, Cella D, Isitt JJ, Wu AW, Young J. 2008; Health-related quality of life of immune thrombocytopenic purpura patients: results from a web-based survey. Curr Med Res Opin. 24:2767–76. DOI: 10.1185/03007990802377461. PMID: 18715526.
Article17. Kühne T, Buchanan GR, Zimmerman S, et al. 2003; A prospective comparative study of 2540 infants and children with newly diagnosed idiopathic thrombocytopenic purpura (ITP) from the Intercontinental Childhood ITP Study Group. J Pediatr. 143:605–8. DOI: 10.1067/S0022-3476(03)00535-3. PMID: 14615730.
Article18. Li S, Molony JT, Cetin K, Wasser JS, Altomare I. 2018; Rate of bleeding-related episodes in elderly patients with primary immune thrombocytopenia: a retrospective cohort study. Curr Med Res Opin. 34:209–16. DOI: 10.1080/03007995.2017.1360852. PMID: 28748715.
Article19. Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. 2017; Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 16:620–32. DOI: 10.1016/j.autrev.2017.04.012. PMID: 28428120.
Article20. Jang JH, Kim JY, Mun YC, et al. 2017; Management of immune thrombocytopenia: Korean experts recommendation in 2017. Blood Res. 52:254–63. DOI: 10.5045/br.2017.52.4.254. PMID: 29333401. PMCID: PMC5762735.
Article21. Din B, Wang X, Shi Y, Li Y. 2015; Long-term effect of high-dose dexamethasone with or without low-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol. 133:124–8. DOI: 10.1159/000362529. PMID: 25247485.
Article22. Praituan W, Rojnuckarin P. 2009; Faster platelet recovery by high-dose dexamethasone compared with standard-dose prednisolone in adult immune thrombocytopenia: a prospective randomized trial. J Thromb Haemost. 7:1036–8. DOI: 10.1111/j.1538-7836.2009.03359.x. PMID: 19548911.
Article23. Mashhadi MA, Kaykhaei MA, Sepehri Z, Miri-Moghaddam E. 2012; Single course of high dose dexamethasone is more effective than conventional prednisolone therapy in the treatment of primary newly diagnosed immune thrombocytopenia. Daru. 20:7. DOI: 10.1186/2008-2231-20-7. PMID: 23351609. PMCID: PMC3557140.
Article24. Jacobs P, Wood L, Novitzky N. 1994; Intravenous gammaglobulin has no advantages over oral corticosteroids as primary therapy for adults with immune thrombocytopenia: a prospective randomized clinical trial. Am J Med. 97:55–9. DOI: 10.1016/0002-9343(94)90048-5. PMID: 8030657.
Article25. Wei Y, Ji XB, Wang YW, et al. 2016; High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 127:296–302. quiz 370DOI: 10.1182/blood-2015-07-659656. PMID: 26480931.
Article26. DiFino SM, Lachant NA, Kirshner JJ, Gottlieb AJ. 1980; Adult idiopathic thrombocytopenic purpura. Clinical findings and response to therapy. Am J Med. 69:430–42. DOI: 10.1016/0002-9343(80)90016-9. PMID: 6998293.27. Houwerzijl EJ, Louwes H, Sluiter WJ, Smit JW, Vellenga E, de Wolf JT. 2008; Platelet production rate predicts the response to prednisone therapy in patients with idiopathic thrombocytopenic purpura. Ann Hematol. 87:975–83. DOI: 10.1007/s00277-008-0537-1. PMID: 18690441.
Article28. Matschke J, Müller-Beissenhirtz H, Novotny J, et al. 2016; A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 136:101–7. DOI: 10.1159/000445420. PMID: 27189086.
Article29. Mazzucconi MG, Francesconi M, Fidani P, et al. 1985; Treatment of idiopathic thrombocytopenic purpura (ITP): results of a multicentric protocol. Haematologica. 70:329–36. PMID: 3935532.30. Centurioni R, Braianzoni F, Olivieri A, et al. 1990; Treatment of autoimmune thrombocytopenic purpura. Acta Haematol Pol. 21:139–43. PMID: 2131713.31. Zimmer J, Andrès E, Noel E, Koumarianou A, Blicklé JF, Maloisel F. 2004; Current management of adult idiopathic thrombocytopenic purpura in practice: a cohort study of 201 patients from a single center. Clin Lab Haematol. 26:137–42. DOI: 10.1111/j.1365-2257.2004.00591.x. PMID: 15053808.
Article32. Bizzoni L, Mazzucconi MG, Gentile M, et al. 2006; Idiopathic thrombocytopenic purpura (ITP) in the elderly: clinical course in 178 patients. Eur J Haematol. 76:210–6. DOI: 10.1111/j.1600-0609.2005.00602.x. PMID: 16412138.
Article33. Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. 2016; Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 15:457–65. DOI: 10.1517/14740338.2016.1140743. PMID: 26789102.
Article34. Rhen T, Cidlowski JA. 2005; Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 353:1711–23. DOI: 10.1056/NEJMra050541. PMID: 16236742.
Article35. Schäcke H, Döcke WD, Asadullah K. 2002; Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 96:23–43. DOI: 10.1016/S0163-7258(02)00297-8. PMID: 12441176.
Article36. Mithoowani S, Gregory-Miller K, Goy J, et al. 2016; High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: a systematic review and meta-analysis. Lancet Haematol. 3:e489–96. DOI: 10.1016/S2352-3026(16)30109-0. PMID: 27658982.
Article37. Bae SH, Ryoo HM, Lee WS, et al. 2010; High dose dexamethasone vs. conventional dose prednisolone for adults with immune thrombocytopenia: a prospective multicenter phase III trial. Blood (ASH Annual Meeting Abstracts). 116(Suppl):3687. DOI: 10.1182/blood.V116.21.3687.3687.
Article38. Li Z, Mou W, Lu G, et al. 2011; Low-dose rituximab combined with short-term glucocorticoids up-regulates Treg cell levels in patients with immune thrombocytopenia. Int J Hematol. 93:91–8. DOI: 10.1007/s12185-010-0753-z. PMID: 21188563.
Article39. Zaja F, Baccarani M, Mazza P, et al. 2010; Dexamethasone plus rituximab yields higher sustained response rates than dexamethasone monotherapy in adults with primary immune thrombocytopenia. Blood. 115:2755–62. DOI: 10.1182/blood-2009-07-229815. PMID: 20130241.40. Gudbrandsdottir S, Birgens HS, Frederiksen H, et al. 2013; Rituximab and dexamethasone vs dexamethasone monotherapy in newly diagnosed patients with primary immune thrombocytopenia. Blood. 121:1976–81. DOI: 10.1182/blood-2012-09-455691. PMID: 23293082.
Article41. Cooper K, Matcham J, Helme K, Akehurst R. 2014; Update on romiplostim and eltrombopag indirect comparison. Int J Technol Assess Health Care. 30:129–30. DOI: 10.1017/S0266462313000767. PMID: 24485057.
Article42. Cooper KL, Fitzgerald P, Dillingham K, Helme K, Akehurst R. 2012; Romiplostim and eltrombopag for immune thrombocytopenia: methods for indirect comparison. Int J Technol Assess Health Care. 28:249–58. DOI: 10.1017/S0266462312000414. PMID: 22980701.
Article43. Wang L, Gao Z, Chen XP, et al. 2016; Efficacy and safety of thrombopoietin receptor agonists in patients with primary immune thrombocytopenia: a systematic review and meta-analysis. Sci Rep. 6:39003. DOI: 10.1038/srep39003. PMID: 27991534. PMCID: PMC5171907.
Article44. Neunert C, Terrell DR, Arnold DM, et al. 2019; American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 3:3829–66. DOI: 10.1182/bloodadvances.2019000966. PMID: 31794604. PMCID: PMC6963252.
Article45. Chater C, Terriou L, Duhamel A, et al. 2016; Reemergence of splenectomy for ITP second-line treatment? Ann Surg. 264:772–7. DOI: 10.1097/SLA.0000000000001912. PMID: 27741009.
Article46. Kojouri K, Vesely SK, Terrell DR, George JN. 2004; Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 104:2623–34. DOI: 10.1182/blood-2004-03-1168. PMID: 15217831.
Article47. Wang T, Xu M, Ji L, Han ZC, Yang R. 2005; Splenectomy for adult chronic idiopathic thrombocytopenic purpura: experience from a single center in China. Eur J Haematol. 75:424–9. DOI: 10.1111/j.1600-0609.2005.00517.x. PMID: 16191093.
Article48. Vianelli N, Galli M, de Vivo A, et al. 2005; Efficacy and safety of splenectomy in immune thrombocytopenic purpura: long-term results of 402 cases. Haematologica. 90:72–7. PMID: 15642672.49. Sampath S, Meneghetti AT, MacFarlane JK, Nguyen NH, Benny WB, Panton ON. 2007; An 18-year review of open and laparoscopic splenectomy for idiopathic thrombocytopenic purpura. Am J Surg. 193:580–3. discussion 583–4. DOI: 10.1016/j.amjsurg.2007.02.002. PMID: 17434359.
Article50. Gonzalez-Porras JR, Escalante F, Pardal E, et al. 2013; Safety and efficacy of splenectomy in over 65-yrs-old patients with immune thrombocytopenia. Eur J Haematol. 91:236–41. DOI: 10.1111/ejh.12146. PMID: 23679653.
Article51. Ahmed R, Devasia AJ, Viswabandya A, et al. 2016; Long-term outcome following splenectomy for chronic and persistent immune thrombocytopenia (ITP) in adults and children: splenectomy in ITP. Ann Hematol. 95:1429–34. DOI: 10.1007/s00277-016-2738-3. PMID: 27370992.
Article52. Zheng CX, Zheng D, Chen LH, Yu JF, Wu ZM. 2011; Laparoscopic splenectomy for immune thrombocytopenic purpura at a teaching institution. Chin Med J (Engl). 124:1175–80. PMID: 21542991.53. Guan Y, Wang S, Xue F, et al. 2017; Long-term results of splenectomy in adult chronic immune thrombocytopenia. Eur J Haematol. 98:235–41. DOI: 10.1111/ejh.12821. PMID: 27753191.
Article54. Park YH, Yi HG, Kim CS, et al. 2016; Clinical outcome and predictive factors in the response to splenectomy in elderly patients with primary immune thrombocytopenia: a multicenter retrospective study. Acta Haematol. 135:162–71. DOI: 10.1159/000442703. PMID: 26771656.
Article55. Li HQ, Zhang L, Zhao H, Ji LX, Yang RC. 2005; Chronic idiopathic thrombocytopenic purpura in adult Chinese patients: a retrospective single-centered analysis of 1791 cases. Chin Med J (Engl). 118:34–7. PMID: 15642223.56. Montalvo J, Velazquez D, Pantoja JP, Sierra M, López-Karpovitch X, Herrera MF. 2014; Laparoscopic splenectomy for primary immune thrombocytopenia: clinical outcome and prognostic factors. J Laparoendosc Adv Surg Tech A. 24:466–70. DOI: 10.1089/lap.2013.0267. PMID: 24905792.
Article57. Balagué C, Vela S, Targarona EM, et al. 2006; Predictive factors for successful laparoscopic splenectomy in immune thrombocytopenic purpura: study of clinical and laboratory data. Surg Endosc. 20:1208–13. DOI: 10.1007/s00464-005-0445-6. PMID: 16865623.
Article58. Wang T, Zhao H, Ren H, et al. 2005; Type 1 and type 2 T-cell profiles in idiopathic thrombocytopenic purpura. Haematologica. 90:914–23. PMID: 15996929.59. Zheng D, Huang CS, Huang SB, Zheng CX. 2016; Laparoscopic splenectomy for primary immune thrombocytopenia: current status and challenges. World J Gastrointest Endosc. 8:610–5. DOI: 10.4253/wjge.v8.i17.610. PMID: 27668071. PMCID: PMC5027031.
Article60. Bussel JB, Kuter DJ, George JN, et al. 2006; AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 355:1672–81. DOI: 10.1056/NEJMoa054626. PMID: 17050891.
Article61. Bussel JB, Cheng G, Saleh MN, et al. 2007; Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 357:2237–47. DOI: 10.1056/NEJMoa073275. PMID: 18046028.
Article62. Bussel JB, Provan D, Shamsi T, et al. 2009; Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 373:641–8. DOI: 10.1016/S0140-6736(09)60402-5. PMID: 19231632.
Article63. Shirasugi Y, Ando K, Miyazaki K, et al. 2011; Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized phase III clinical trial. Int J Hematol. 94:71–80. DOI: 10.1007/s12185-011-0886-8. PMID: 21706145.
Article64. Tomiyama Y, Miyakawa Y, Okamoto S, et al. 2012; A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J Thromb Haemost. 10:799–806. DOI: 10.1111/j.1538-7836.2012.04695.x. PMID: 22409309.
Article65. Yang R, Li J, Jin J, et al. 2017; Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Br J Haematol. 176:101–10. DOI: 10.1111/bjh.14380. PMID: 27734464.
Article66. Kuter DJ, Rummel M, Boccia R, et al. 2010; Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 363:1889–99. DOI: 10.1056/NEJMoa1002625. PMID: 21067381.
Article67. Provan D, Arnold DM, Bussel JB, et al. 2019; Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 3:3780–817. DOI: 10.1182/bloodadvances.2019000812. PMID: 31770441. PMCID: PMC6880896.
Article68. Blanchette VS, Luke B, Andrew M, et al. 1993; A prospective, randomized trial of high-dose intravenous immune globulin G therapy, oral prednisone therapy, and no therapy in childhood acute immune thrombocytopenic purpura. J Pediatr. 123:989–95. DOI: 10.1016/S0022-3476(05)80400-7. PMID: 8229536.
Article69. Sartorius JA. 1984; Steroid treatment of idiopathic thrombocytopenic purpura in children. Preliminary results of a randomized cooperative study. Am J Pediatr Hematol Oncol. 6:165–9. DOI: 10.1097/00043426-198406020-00008. PMID: 6540532.70. Fujisawa K, Iyori H, Ohkawa H, et al. 2000; A prospective, randomized trial of conventional, dose-accelerated corticosteroids and intravenous immunoglobulin in children with newly diagnosed idiopathic thrombocytopenic purpura. Int J Hematol. 72:376–83. PMID: 11185998.71. Blanchette V, Imbach P, Andrew M, et al. 1994; Randomised trial of intravenous immunoglobulin G, intravenous anti-D, and oral prednisone in childhood acute immune thrombocytopenic purpura. Lancet. 344:703–7. DOI: 10.1016/S0140-6736(94)92205-5. PMID: 7915773.
Article72. Imbach P, Wagner HP, Berchtold W, et al. 1985; Intravenous immunoglobulin versus oral corticosteroids in acute immune thrombocytopenic purpura in childhood. Lancet. 2:464–8. DOI: 10.1016/S0140-6736(85)90400-3. PMID: 2863492.
Article73. Celik M, Bulbul A, Aydogan G, et al. 2013; Comparison of anti-D immunoglobulin, methylprednisolone, or intravenous immunoglobulin therapy in newly diagnosed pediatric immune thrombocytopenic purpura. J Thromb Thrombolysis. 35:228–33. DOI: 10.1007/s11239-012-0801-z. PMID: 22956408.
Article74. Bussel JB, Buchanan GR, Nugent DJ, et al. 2011; A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 118:28–36. DOI: 10.1182/blood-2010-10-313908. PMID: 21502541.
Article75. Grainger JD, Locatelli F, Chotsampancharoen T, et al. 2015; Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): a randomised, multicentre, placebo-controlled trial. Lancet. 386:1649–58. DOI: 10.1016/S0140-6736(15)61107-2. PMID: 26231455.
Article76. Elalfy MS, Abdelmaksoud AA, Eltonbary KY. 2011; Romiplostim in children with chronic refractory ITP: randomized placebo controlled study. Ann Hematol. 90:1341–4. DOI: 10.1007/s00277-011-1172-9. PMID: 21318572.
Article77. González-López TJ, Pascual C, Álvarez-Román MT, et al. 2015; Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am J Hematol. 90:E40–3. DOI: 10.1002/ajh.23900. PMID: 25400215.
Article78. Iino M, Sakamoto Y, Sato T. 2020; Treatment-free remission after thrombopoietin receptor agonist discontinuation in patients with newly diagnosed immune thrombocytopenia: an observational retrospective analysis in real-world clinical practice. Int J Hematol. 112:159–68. DOI: 10.1007/s12185-020-02893-y. PMID: 32476083.
Article79. Newland A, Godeau B, Priego V, et al. 2016; Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 172:262–73. DOI: 10.1111/bjh.13827. PMID: 26537623.
Article80. Ghanima W, Khelif A, Waage A, et al. 2015; Rituximab as second-line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomized, double-blind, placebo-controlled trial. Lancet. 385:1653–61. DOI: 10.1016/S0140-6736(14)61495-1.81. Poudyal BS, Sapkota B, Shrestha GS, Thapalia S, Gyawali B, Tuladhar S. 2016; Safety and efficacy of azathioprine as a second line therapy for primary immune thrombocytopenic purpura. JNMA J Nepal Med Assoc. 55:16–21. DOI: 10.31729/jnma.2832. PMID: 27935917.
Article82. Verlin M, Laros RK Jr, Penner JA. 1976; Treatment of refractory thrombocytopenic purpura with cyclophosphamine. Am J Hematol. 1:97–104. DOI: 10.1002/ajh.2830010111. PMID: 988746.83. Choudhary DR, Naithani R, Mahapatra M, Kumar R, Mishra P, Saxena R. 2008; Efficacy of cyclosporine as a single agent therapy in chronic idiopathic thrombocytopenic purpura. Haematologica. 93:e61–2. discussion e63DOI: 10.3324/haematol.13481. PMID: 18827257.
Article84. Kim SW, Rice L, McCarthy JJ. 1997; Efficacy of danazol with autoimmune thrombocytopenia. Clin Appl Thromb Hemost. 3:251–5. DOI: 10.1177/107602969700300406.
Article85. Damodar S, Viswabandya A, George B, Mathews V, Chandy M, Srivastava A. 2005; Dapsone for chronic idiopathic thrombocytopenic purpura in children and adults--a report on 90 patients. Eur J Haematol. 75:328–31. DOI: 10.1111/j.1600-0609.2005.00545.x. PMID: 16146539.
Article86. Godeau B, Durand JM, Roudot-Thoraval F, et al. 1997; Dapsone for chronic autoimmune thrombocytopenic purpura: a report of 66 cases. Br J Haematol. 97:336–9. DOI: 10.1046/j.1365-2141.1997.412687.x. PMID: 9163598.
Article87. Patel AP, Patil AS. 2015; Dapsone for immune thrombocytopenic purpura in children and adults. Platelets. 26:164–7. DOI: 10.3109/09537104.2014.886677. PMID: 24512442.
Article88. Vancine-Califani SM, De Paula EV, Ozelo MC, Orsi FL, Fabri DR, Annichino-Bizzacchi JM. 2008; Efficacy and safety of dapsone as a second-line treatment in non-splenectomized adults with immune thrombocytopenic purpura. Platelets. 19:489–95. DOI: 10.1080/09537100802315110. PMID: 18979360.
Article89. Zaja F, Marin L, Chiozzotto M, Puglisi S, Volpetti S, Fanin R. 2012; Dapsone salvage therapy for adult patients with immune thrombocytopenia relapsed or refractory to steroid and rituximab. Am J Hematol. 87:321–3. DOI: 10.1002/ajh.22266. PMID: 22190262.
Article90. Hou M, Peng J, Shi Y, et al. 2003; Mycophenolate mofetil (MMF) for the treatment of steroid-resistant idiopathic thrombocytopenic purpura. Eur J Haematol. 70:353–7. DOI: 10.1034/j.1600-0609.2003.00076.x. PMID: 12756016.
Article91. Miano M, Ramenghi U, Russo G, et al. 2016; Mycophenolate mofetil for the treatment of children with immune thrombocytopenia and Evans syndrome. A retrospective data review from the Italian association of paediatric haematology/oncology. Br J Haematol. 175:490–5. DOI: 10.1111/bjh.14261. PMID: 27447678.
Article92. Taylor A, Neave L, Solanki S, et al. 2015; Mycophenolate mofetil therapy for severe immune thrombocytopenia. Br J Haematol. 171:625–30. DOI: 10.1111/bjh.13622. PMID: 26250874.
Article93. Ahn YS, Harrington WJ, Seelman RC, Eytel CS. 1974; Vincristine therapy of idiopathic and secondary thrombocytopenias. N Engl J Med. 291:376–80. DOI: 10.1056/NEJM197408222910802. PMID: 4847353.
Article94. Fresneau B, Petit A, Courcoux MF, et al. 2011; Vinblastine in the treatment of children and adolescents with refractory immune thrombocytopenia. Am J Hematol. 86:785–7. DOI: 10.1002/ajh.22081. PMID: 21800354.
Article95. Park YH, Yi HG, Lee MH, Kim CS, Lim JH. 2016; Clinical efficacy and tolerability of vincristine in splenectomized patients with refractory or relapsed immune thrombocytopenia: a retrospective single-center study. Int J Hematol. 103:180–8. DOI: 10.1007/s12185-015-1903-0. PMID: 26588926.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and management of thrombocytopenia in pregnancy
- Diagnostic Approach of Childhood Immune Thrombocytopenia
- Advances in management of pediatric chronic immune thrombocytopenia: a narrative review
- Quick drop of platelet counts in children with chronic immune thrombocytopenia after COVID-19 mRNA vaccination: case reports
- Thrombosis and severe acute respiratory syndrome coronavirus 2 vaccines: vaccine-induced immune thrombotic thrombocytopenia