J Korean Med Sci.
2022 Mar;37(11):e90. 10.3346/jkms.2022.37.e90.
Growth Responses During 3 Years of Growth Hormone Treatment in Children and Adolescents With Growth Hormone Deficiency: Comparison Between Idiopathic, Organic and Isolated Growth Hormone Deficiency, and Multiple Pituitary Hormone Deficiency
- Affiliations
-
- 1Department of Pediatrics, Chungnam National University College of Medicine, Daejeon, Korea
- 2Lee Gyung Min Pediatric Clinic, Daejeon, Korea
- 3Joey Children’s Hospital, Daejeon, Korea
- 4Department of Pediatrics, Chungbuk National University College of Medicine, Cheongju, Korea
- 5Department of Pediatrics, Dankook University College of Medicine, Cheonan, Korea
- KMID: 2527450
- DOI: http://doi.org/10.3346/jkms.2022.37.e90
Abstract
- Background
The study aimed to compare the growth responses to 3 years of growth hormone (GH) treatment in children and adolescents with GH deficiency (GHD) according to idiopathic, organic, isolated (IGHD), and multiple pituitary hormone deficiency (MPHD).
Methods
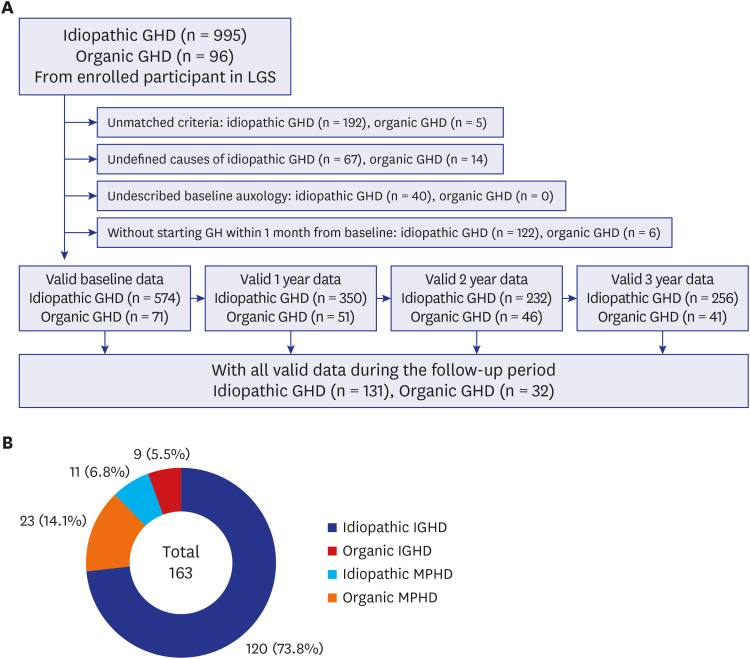
Total 163 patients aged 2–18 years (100 males and 63 females; 131 idiopathic and 32 organic GHD; 129 IGHD and 34 MPHD) were included from data obtained from the LG Growth Study. Parameters of growth responses and biochemical results were compared during the 3-year GH treatment.
Results
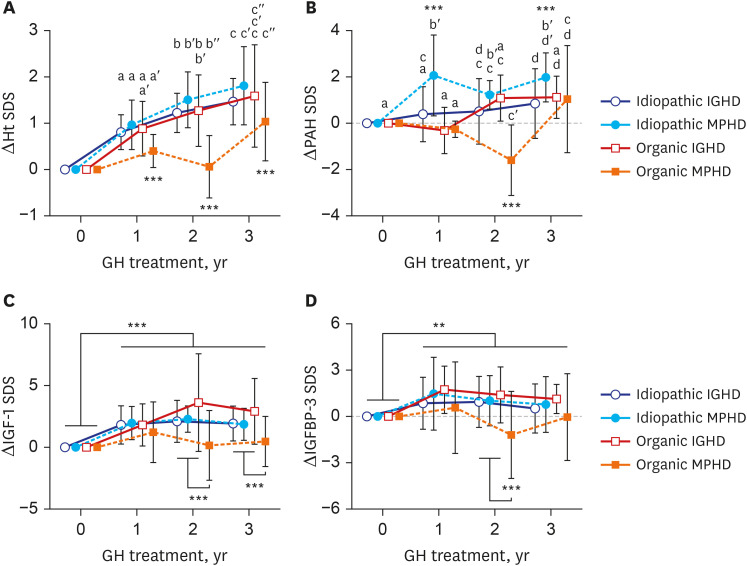
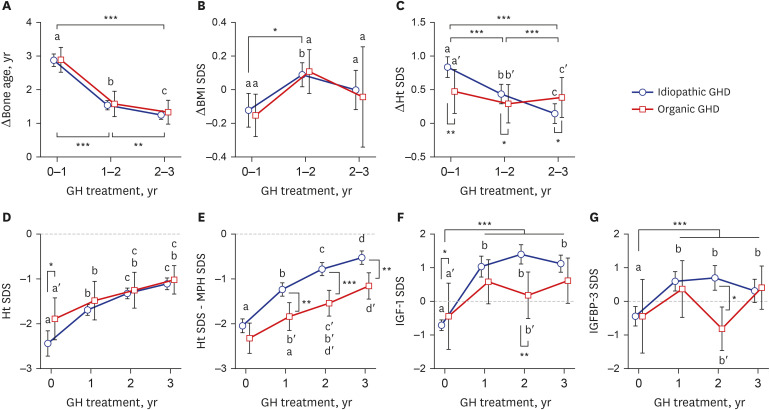
The baseline age, bone age (BA), height (Ht) standard deviation score (SDS), weight SDS, mid-parental Ht SDS, predicted adult Ht (PAH) SDS, and insulin like growth factor-1 (IGF-1) SDS were significantly higher in the organic GHD patients than in the idiopathic GHD patients, but peak GH on the GH-stimulation test, baseline GH dose, and mean 3-year-GH dosage were higher in the idiopathic GHD patients than in the organic GHD patients. The prevalence of MPHD was higher in the organic GHD patients than in the idiopathic GHD patients. Idiopathic MPHD subgroup showed the largest increase for the ΔHt SDS and ΔPAH SDS during GH treatment, and organic MPHD subgroup had the smallest mean increase after GH treatment, depending on ΔIGF-1 SDS and ΔIGF binding protein-3 (IGFBP-3) SDS. The growth velocity and the parental-adjusted Ht gain were greater in the idiopathic GHD patients than the organic GHD patients during the 3-year GH treatment, which may have been related to the different GH dose, ΔIGF-1 SDS, and ΔIGFBP-3 SDS between two groups. Multiple linear regression analysis revealed that baseline IGF-1 SDS, BA, and MPH SDS in idiopathic group and baseline HT SDS in organic group are the most predictable parameters for favorable 3-year-GH treatment.
Conclusion
The 3-year-GH treatment was effective in both idiopathic and organic GHD patients regardless of the presence of MPHD or underlying causes, but their growth outcomes were not constant with each other. Close monitoring along with appropriate dosage of GH and annual growth responses, not specific at baseline, are more important in children and adolescents with GHD for long-term treatment.
Keyword
Figure
Reference
-
1. Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994; 125(1):29–35. PMID: 8021781.2. Boguszewski MCS. Growth hormone deficiency and replacement in children. Rev Endocr Metab Disord. 2021; 22(1):101–108. PMID: 33029711.3. Pozzobon G, Partenope C, Mora S, Garbetta G, Weber G, Barera G. Growth hormone therapy in children: predictive factors and short-term and long-term response criteria. Endocrine. 2019; 66(3):614–621. PMID: 31423546.4. Kriström B, Aronson AS, Dahlgren J, Gustafsson J, Halldin M, Ivarsson SA, et al. Growth hormone (GH) dosing during catch-up growth guided by individual responsiveness decreases growth response variability in prepubertal children with GH deficiency or idiopathic short stature. J Clin Endocrinol Metab. 2009; 94(2):483–490. PMID: 19001519.5. Bakker B, Frane J, Anhalt H, Lippe B, Rosenfeld RG. Height velocity targets from the national cooperative growth study for first-year growth hormone responses in short children. J Clin Endocrinol Metab. 2008; 93(2):352–357. PMID: 18000092.6. Ranke MB, Lindberg A. KIGS International Board. Observed and predicted growth responses in prepubertal children with growth disorders: guidance of growth hormone treatment by empirical variables. J Clin Endocrinol Metab. 2010; 95(3):1229–1237. PMID: 20097713.7. Child CJ, Blum WF, Deal C, Zimmermann AG, Quigley CA, Drop SL, et al. Development of additional pituitary hormone deficiencies in pediatric patients originally diagnosed with isolated growth hormone deficiency due to organic causes. Eur J Endocrinol. 2016; 174(5):669–679. PMID: 26888628.8. Straetemans S, Roelants M, Thomas M, Rooman R, De Schepper J. Reference curve for the first-year growth response to growth hormone treatment in prepubertal children with idiopathic growth hormone deficiency: validation of the KIGS first-year growth response curve using the Belgian Register for the Study of Growth and Puberty Problems. Horm Res Paediatr. 2014; 81(5):343–349. PMID: 24686034.9. Milner RD, Russell-Fraser T, Brook CG, Cotes PM, Farquhar JW, Parkin JM, et al. Experience with human growth hormone in Great Britain: the report of the MRC Working Party. Clin Endocrinol (Oxf). 1979; 11(1):15–38. PMID: 229995.10. Herber SM, Dunsmore IR, Milner RD. Final stature in brain tumours other than craniopharyngioma: effect of growth hormone. Horm Res. 1985; 22(1-2):63–67. PMID: 4029881.11. Chung S, Yoo JH, Choi JH, Rhie YJ, Chae HW, Kim JH, et al. Design of the long-term observational cohort study with recombinant human growth hormone in Korean children: LG Growth Study. Ann Pediatr Endocrinol Metab. 2018; 23(1):43–50. PMID: 29609449.12. Wyatt DT, Mark D, Slyper A. Survey of growth hormone treatment practices by 251 pediatric endocrinologists. J Clin Endocrinol Metab. 1995; 80(11):3292–3297. PMID: 7593441.13. Korean Society of Pediatric Endocrinology. Pediatric Endocrinology. 3rd ed. Paju, Korea: KOONJA Publishing Inc.;2014.14. Murray PG, Dattani MT, Clayton PE. Controversies in the diagnosis and management of growth hormone deficiency in childhood and adolescence. Arch Dis Child. 2016; 101(1):96–100. PMID: 26153506.15. Rhee N, Oh KY, Yang EM, Kim CJ. Growth hormone responses to provocative tests in children with short stature. Chonnam Med J. 2015; 51(1):33–38. PMID: 25914878.16. Kim JH, Yun S, Hwang SS, Shim JO, Chae HW, Lee YJ, et al. The 2017 Korean National Growth Charts for children and adolescents: development, improvement, and prospects. Korean J Pediatr. 2018; 61(5):135–149. PMID: 29853938.17. Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952; 40(4):423–441. PMID: 14918032.18. Hyun SE, Lee BC, Suh BK, Chung SC, Ko CW, Kim HS, et al. Reference values for serum levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in Korean children and adolescents. Clin Biochem. 2012; 45(1-2):16–21. PMID: 22032863.19. Blum WF, Deal C, Zimmermann AG, Shavrikova EP, Child CJ, Quigley CA, et al. Development of additional pituitary hormone deficiencies in pediatric patients originally diagnosed with idiopathic isolated GH deficiency. Eur J Endocrinol. 2013; 170(1):13–21. PMID: 24088548.20. Stanhope R, De Luca F, Delemarre-Van de Waal HA, Liotta A, Norjavaara E, Salvatoni A, et al. Multiple pituitary hormone deficiency: management of puberty for optimal auxological results. J Pediatr Endocrinol Metab. 2001; 14(Suppl 2):1009–1014. PMID: 11529397.21. Darendeliler F, Lindberg A, Wilton P. Response to growth hormone treatment in isolated growth hormone deficiency versus multiple pituitary hormone deficiency. Horm Res Paediatr. 2011; 76(Suppl 1):42–46.22. Maghnie M, Ambrosini L, Cappa M, Pozzobon G, Ghizzoni L, Ubertini MG, et al. Adult height in patients with permanent growth hormone deficiency with and without multiple pituitary hormone deficiencies. J Clin Endocrinol Metab. 2006; 91(8):2900–2905. PMID: 16684828.23. Reiter EO, Price DA, Wilton P, Albertsson-Wikland K, Ranke MB. Effect of growth hormone (GH) treatment on the near-final height of 1258 patients with idiopathic GH deficiency: analysis of a large international database. J Clin Endocrinol Metab. 2006; 91(6):2047–2054. PMID: 16537676.24. Huang YH, Wai YY, Van YH, Lo FS. Effect of growth hormone therapy on Taiwanese children with growth hormone deficiency. J Formos Med Assoc. 2012; 111(7):355–363. PMID: 22817812.25. Blethen SL, Compton P, Lippe BM, Rosenfeld RG, August GP, Johanson A. Factors predicting the response to growth hormone (GH) therapy in prepubertal children with GH deficiency. J Clin Endocrinol Metab. 1993; 76(3):574–579. PMID: 8445013.26. Stanley T. Diagnosis of growth hormone deficiency in childhood. Curr Opin Endocrinol Diabetes Obes. 2012; 19(1):47–52. PMID: 22157400.27. Choi IJ, Hwang JS, Shin CH, Yang SW. Factors affecting on final adult height and total height gain in children with idiopathic and organic growth hormone deficiency after growth hormone treatment. J Korean Pediatr Soc. 2003; 46(8):803–810.28. Rachmiel M, Rota V, Atenafu E, Daneman D, Hamilton J. Final height in children with idiopathic growth hormone deficiency treated with a fixed dose of recombinant growth hormone. Horm Res. 2007; 68(5):236–243. PMID: 17396034.29. Hughes IP, Harris M, Choong CS, Ambler G, Cutfield W, Hofman P, et al. Growth hormone regimens in Australia: analysis of the first 3 years of treatment for idiopathic growth hormone deficiency and idiopathic short stature. Clin Endocrinol (Oxf). 2012; 77(1):62–71. PMID: 21950731.30. Cutfield WS, Lundgren F. Insulin-like growth factor I and growth responses during the first year of growth hormone treatment in KIGS patients with idiopathic growth hormone deficiency, acquired growth hormone deficiency, turner syndrome and born small for gestational age. Horm Res. 2009; 71(Suppl 1):39–45. PMID: 19153504.31. Vassilopoulou-Sellin , Klein MJ, Moore BD 3rd, Reid HL, Ater J, Zietz HA. Efficacy of growth hormone replacement therapy in children with organic growth hormone deficiency after cranial irradiation. Horm Res. 1995; 43(5):188–193. PMID: 7782048.32. Karavanaki K, Kontaxaki C, Maniati-Christidi M, Petrou V, Dacou-Voutetakis C. Growth response, pubertal growth and final height in Greek children with growth hormone (GH) deficiency on long-term GH therapy and factors affecting outcome. J Pediatr Endocrinol Metab. 2001; 14(4):397–405. PMID: 11327373.33. Kriström B, Lundberg E, Jonsson B, Albertsson-Wikland K; study group. IGF-1 and growth response to adult height in a randomized GH treatment trial in short non-GH-deficient children. J Clin Endocrinol Metab. 2014; 99(8):2917–2924. PMID: 24823461.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical effects of yeast derived recombinant methionyl growth hormone in growth hormone deficiency
- Effect of yeast-derived methionyl recombinant growth hormone on growth hormone deficient dwarf
- Quantification of human urinary growth hormone and its clinical significance in the diagnosis of growth hormone deficiency
- Growth Response in Children with Idiopathic Growth Hormone Deficiency and Organic Growth Hormone Deficiency during Growth Hormone Treatment
- A Case of X-linked Agammaglobulinemia with Delayed Growth