J Korean Foot Ankle Soc.
2022 Mar;26(1):48-53. 10.14193/jkfas.2022.26.1.48.
Effect of Severe Limb Purpura Following the Administration of COVID-19 Vaccination on a Diabetic Foot Requiring Amputation: A Case Report
- Affiliations
-
- 1Departments of Orthopaedic Surgery, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 2Departments of Pathology, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- KMID: 2527012
- DOI: http://doi.org/10.14193/jkfas.2022.26.1.48
Abstract
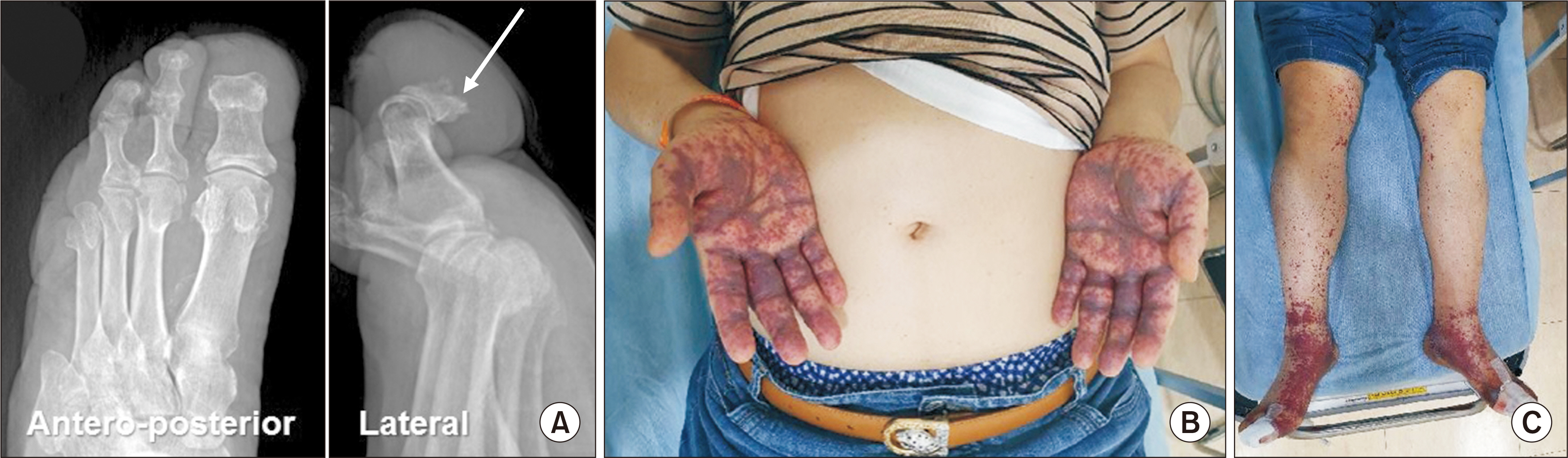
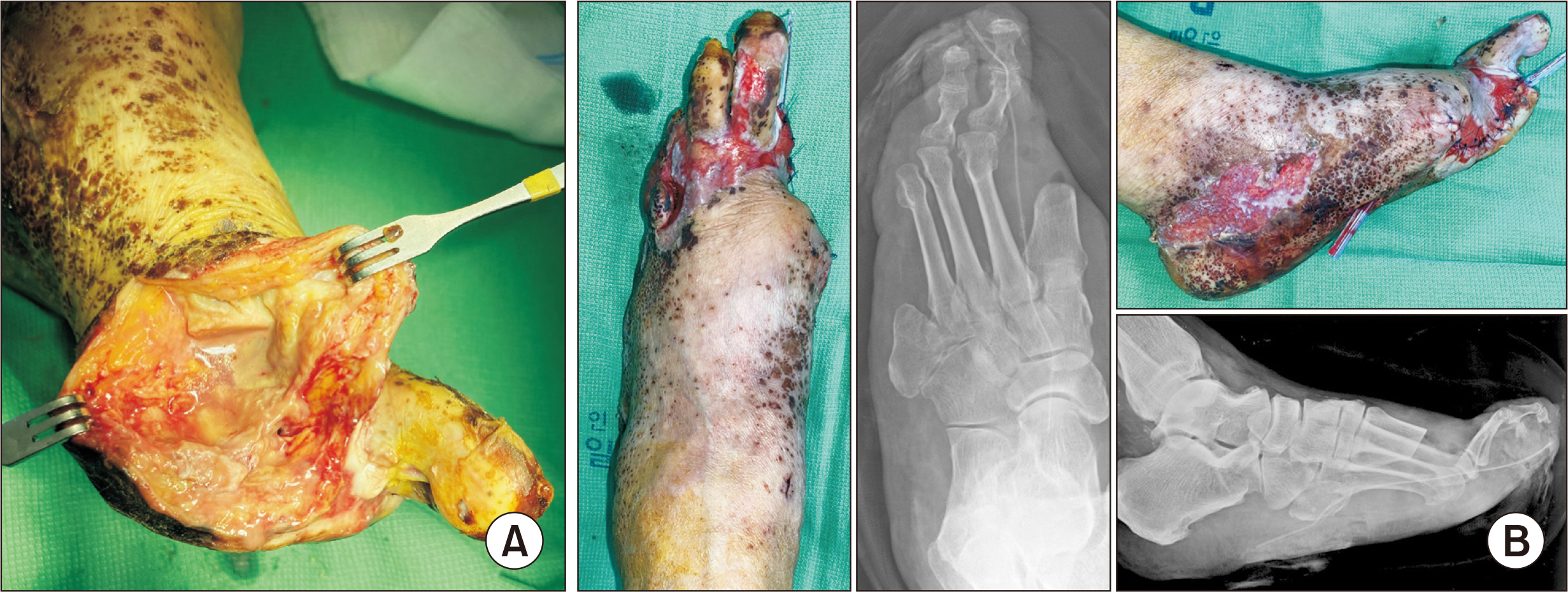
- The current SARS-CoV‑2 coronavirus disease 2019 (COVID-19) pandemic has been a particular challenge for diabetes patients. Since these patients are at a higher risk of COVID-19, they have been prioritized for vaccination. In this report, we describe the case of a patient scheduled for diabetic foot amputation who received the first dose of ChAdOx1 nCov-19 vaccine and subsequently developed severe purpura in his genitalia and both of his hands and feet, accompanied by acute renal failure. The operation had to be postponed as severe limb purpura appeared just before the operation. With adequate management for acute renal failure and topical steroid application for the severe purpura lesions, a successful outcome could be obtained after the delayed first ray amputation. We recommend that COVID-19 vaccination should be carefully administered in patients with a diabetic foot requiring amputation.
Keyword
Figure
Reference
-
1. Pormohammad A, Zarei M, Ghorbani S, Mohammadi M, Razizadeh MH, Turner DL, et al. 2021; Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel). 9:467. doi: 10.3390/vaccines9050467. DOI: 10.3390/vaccines9050467. PMID: 34066475. PMCID: PMC8148145.
Article2. Forni G, Mantovani A. 2021; COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 28:626–39. doi: 10.1038/s41418-020-00720-9. DOI: 10.1038/s41418-020-00720-9. PMID: 33479399. PMCID: PMC7818063.
Article3. World Health Organization. 2021. COVID-19 vaccine tracker and landscape. World Health Organization;Geneva:4. Cines DB, Bussel JB. 2021; SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 384:2254–6. doi: 10.1056/NEJMe2106315. DOI: 10.1056/NEJMe2106315. PMID: 33861524. PMCID: PMC8063912.
Article5. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. 2021; Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 384:2092–101. doi: 10.1056/NEJMoa2104840. DOI: 10.1056/NEJMoa2104840. PMID: 33835769. PMCID: PMC8095372.
Article6. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. 2021; Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 384:2124–30. DOI: 10.1056/NEJMoa2104882. PMID: 33835768. PMCID: PMC8112568.
Article7. Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, et al. 2021; Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 373:n1114. doi: 10.1136/bmj.n1114. DOI: 10.1136/bmj.n1114. PMID: 33952445. PMCID: PMC8097496.
Article8. Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. 2021; Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 384:2202–11. doi: 10.1056/NEJMoa2105385. DOI: 10.1056/NEJMoa2105385. PMID: 33861525. PMCID: PMC8112532.
Article9. Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. 2021; First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 27:1290–7. doi: 10.1038/s41591-021-01408-4. DOI: 10.1038/s41591-021-01408-4. PMID: 34108714. PMCID: PMC8282499.
Article10. Kharkar V, Vishwanath T, Mahajan S, Joshi R, Gole P. 2021; Asymmetrical cutaneous vasculitis following COVID-19 vaccination with unusual eosinophil preponderance. Clin Exp Dermatol. 46:1596–7. doi: 10.1111/ced.14797. DOI: 10.1111/ced.14797. PMID: 34115904. PMCID: PMC8444878.
Article11. Cohen SR, Prussick L, Kahn JS, Gao DX, Radfar A, Rosmarin D. 2021; Leukocytoclastic vasculitis flare following the COVID-19 vaccine. Int J Dermatol. 60:1032–3. doi: 10.1111/ijd.15623. DOI: 10.1111/ijd.15623. PMID: 33928638. PMCID: PMC8239799.
Article12. Bostan E, Gulseren D, Gokoz O. 2021; New-onset leukocytoclastic vasculitis after COVID-19 vaccine. Int J Dermatol. 60:1305–6. doi: 10.1111/ijd.15777. DOI: 10.1111/ijd.15777. PMID: 34241833. PMCID: PMC8444793.
Article13. Watanabe T. 2017; Vasculitis following influenza vaccination: a review of the literature. Curr Rheumatol Rev. 13:188–96. doi: 10.2174/1573397113666170517155443. DOI: 10.2174/1573397113666170517155443. PMID: 28521688.
Article14. Bonetto C, Trotta F, Felicetti P, Alarcón GS, Santuccio C, Bachtiar NS, et al. 2016; Vasculitis as an adverse event following immunization - systematic literature review. Vaccine. 34:6641–51. doi: 10.1016/j.vaccine.2015.09.026. DOI: 10.1016/j.vaccine.2015.09.026. PMID: 26398442.
Article15. Guimarães LE, Baker B, Perricone C, Shoenfeld Y. 2015; Vaccines, adjuvants and autoimmunity. Pharmacol Res. 100:190–209. doi: 10.1016/j.phrs.2015.08.003. DOI: 10.1016/j.phrs.2015.08.003. PMID: 26275795. PMCID: PMC7129276.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amputation in Diabetic Foot Ulcer and Infection
- Surgical Treatment of Diabetic Foot Disease
- Daily Life Management Guidelines for Diabetic Foot Patients
- Incidence and Risk Factors of Ipsilateral Foot and Lower Limb Reamputation in Diabetic Foot Patients
- Multiple Abscesses Following COVID-19 Vaccination: A Case Report