J Korean Med Sci.
2022 Mar;37(9):e73. 10.3346/jkms.2022.37.e73.
Clinical Features of Patients Presenting to the Emergency Department With Cardiovascular Adverse Reactions After COVID-19 mRNA Vaccination
- Affiliations
-
- 1Department of Emergency Medicine, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Incheon, Korea
- 2Department of Emergency Medicine, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea
- 3Department of Emergency Medicine, David Geffen School of Medicine at UCLA, VA Greater Los Angeles Healthcare System, Los Angeles, CA, USA
- KMID: 2526971
- DOI: http://doi.org/10.3346/jkms.2022.37.e73
Abstract
- Background
Since the implementation of the nationwide coronavirus disease 2019 (COVID-19) vaccination campaign, emergency departments (EDs) have had an increasing number of patients reporting postvaccination cardiovascular adverse effects. We investigated the clinical features of patients who visited the ED for cardiovascular adverse reactions after COVID-19 mRNA vaccination.
Methods
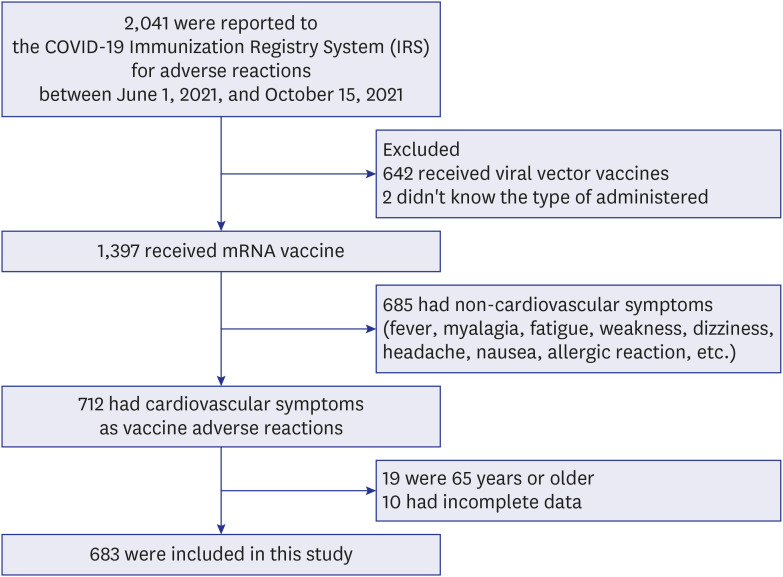
We conducted a retrospective observational study in two EDs. Patients with cardiovascular adverse reactions after COVID-19 mRNA vaccination who visited EDs between June 1, 2021, and October 15, 2021, were selected. The clinical data of these patients were collected by reviewing medical records.
Results
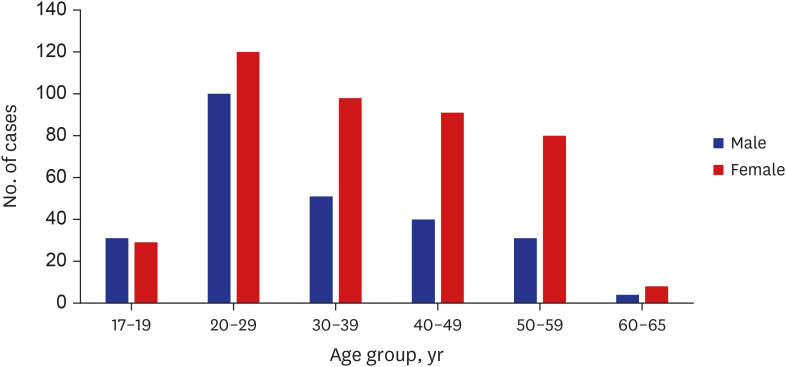
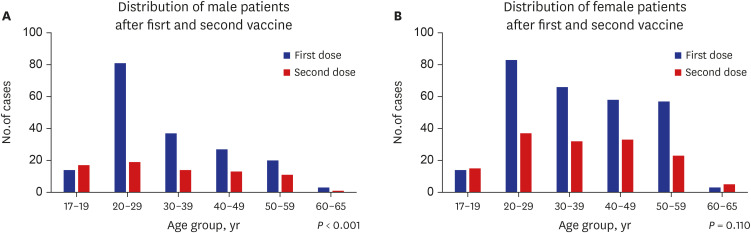
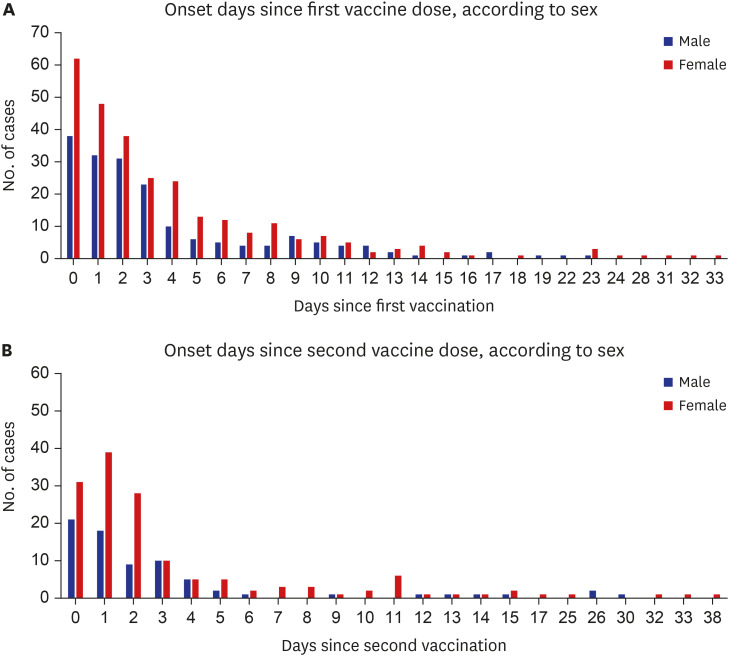
Among 683 patients, 426 (62.4%) were female. The number of patients in their 20s was the highest (38.9% of males, 28.2% of females) (P < 0.001). More patients visited the ED for adverse reactions following the first vaccine dose than following the second dose (67.6% vs. 32.2%). Chief complaints were chest pain/discomfort (74.4%), dyspnea (14.3%) and palpitation (11.3%). The final diagnosis was a nonspecific cause (63.1%), and 663 (97.1%) patients were discharged from the ED. The admission rate was higher in males than in females (3.9% vs. 1.9%). Myocarditis was diagnosed in four males, who showed mild clinical progression and were discharged within 5 hospital days.
Conclusion
Most patients who visited the ED with cardiovascular adverse reactions were discharged from the ED, but some were admitted for other medical diseases as well as adverse vaccine reactions. Therefore, further surveillance and a differential diagnosis of cardiovascular adverse events after COVID-19 mRNA vaccination should be considered by emergency physicians.
Figure
Reference
-
1. Korea Disease Control and Prevention Agency. Criteria for Covid-19 vaccination (revised 211001). Updated 2021. Accessed October 5, 2021. https://ncv.kdca.go.kr/board.es?mid=a12101000000&bid=0031 .2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.
Article3. Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021; 385(23):2140–2149. PMID: 34614328.
Article4. Centers for Disease Control and Prevention. Advisory committee on immunization practices (ACIP), COVID-19 vaccine safety updates, June 23, 2021. Updated 2021. Accessed July 6, 2021. http://cdc.gov/coronavirus .5. Kime P. Pentagon tracking 14 cases of heart inflammation in troops after COVID19 shots. 2021. Updated 2021. Accessed May 16, 2021. https://www.military.com/daily-news/2021/04/26/pentagon-tracking-14-cases-of-heart-inflammation-troops-after-covid-19-shots.html .6. Reuters. Israel examining heart inflammation cases in people who received Pfizer COVID shot. Updated 2021. Accessed April 25, 2021. https://www.reuters.com/world/middle-east/israel-examining-heart-inflammation-cases-people-who-received-pfizer-covid-shot-2021-04-25/ .7. Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021; 36(17):e115. PMID: 33942579.
Article8. Lee YW, Lim SY, Lee JH, Lim JS, Kim M, Kwon S, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021; 36(21):e153. PMID: 34060261.
Article9. Korea Disease Control and Prevention Agency. Weekly COVID-19 vaccination adverse event (Week 33). Updated 2021. Accessed October 21, 2021. https://ncv.kdca.go.kr/board.es?mid=a11707010000&bid=0032#content .10. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021; 144(6):471–484. PMID: 34281357.
Article11. Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021; 385(23):2132–2139. PMID: 34614329.
Article12. Kim J, Cho MJ. Acute myocarditis in children: a 10-year nationwide study (2007–2016) based on the Health Insurance Review and Assessment Service Database in Korea. Korean Circ J. 2020; 50(11):1013–1022. PMID: 32812406.
Article13. Arola A, Pikkarainen E, Sipilä JO, Pykäri J, Rautava P, Kytö V. Occurrence and features of childhood myocarditis: a nationwide study in Finland. J Am Heart Assoc. 2017; 6(11):e005306. PMID: 29151030.
Article14. Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015; 386(9995):743–800. PMID: 26063472.15. Park H, Yun KW, Kim KR, Song SH, Ahn B, Kim DR, et al. Epidemiology and clinical features of myocarditis/pericarditis before the introduction of mRNA COVID-19 vaccine in Korean children: a multicenter study. J Korean Med Sci. 2021; 36(32):e232. PMID: 34402230.
Article16. Olejniczak M, Schwartz M, Webber E, Shaffer A, Perry TE. Viral myocarditis-incidence, diagnosis and management. J Cardiothorac Vasc Anesth. 2020; 34(6):1591–1601. PMID: 32127272.
Article17. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP). Coronavirus disease 2019 (COVID-19) vaccines. Updated 2021. Accessed July 6, 2021. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse events and preventive measures related to COVID-19 vaccines
- BNT162b2 mRNA COVID-19 vaccine adverse reactions needing medical support: a vaccine center experience
- Cutaneous Adverse Reaction After COVID-19 Vaccination
- Cases of Pernio-Like Lesions after mRNA-1273 Vaccination with Clinical and Pathological Features: A Single-Center Experience
- COVID-19 Vaccination in Korea