J Korean Med Sci.
2022 Mar;37(9):e55. 10.3346/jkms.2022.37.e55.
Surgically Metabolic Resection of Pericardial Fat to Ameliorate Myocardial Mitochondrial Dysfunction in Acute Myocardial Infarction Obese Rats
- Affiliations
-
- 1Division of Cardiology, College of Medicine, Heart Research Institute and Biomedical Research Institute, Chung-Ang University Hospital, Chung-Ang University, Seoul, Korea
- 2College of Pharmacy, Mokpo National University, Muan, Korea
- 3Division of Cardiothoracic Surgery, College of Medicine, Chung-Ang University Hospital, Chung-Ang University, Seoul, Korea
- 4Department of Biochemistry and Molecular Biology, School of Medicine, Eulji University, Daejeon, Korea
- KMID: 2526967
- DOI: http://doi.org/10.3346/jkms.2022.37.e55
Abstract
- Background
Pericardial fat (PF) is highly associated with cardiovascular disease but the effectiveness of surgical resection of PF is still unknown for myocardial mitochondrial structure and function in acute myocardial infarction (AMI) with obesity. The aim of this study was to demonstrate the difference in myocardial mitochondrial structure and function between obese AMI with additionally resected PF and those without resected PF.
Methods
Obese rats with 12-week high fat diet (45 kcal% fat, n = 21) were randomly assigned into 3 groups: obese control, obese AMI and obese AMI with additionally resected PF. One week after developing AMI and additional resection of PF, echocardiogram, myocardial mitochondrial histomorphology, oxidative phosphorylation system (OXPHOS), anti-oxidative enzyme and sarcoplasmic reticulum Ca 2+ ATPase 2 (SERCA2) in the non-infarcted area were assessed between these groups.
Results
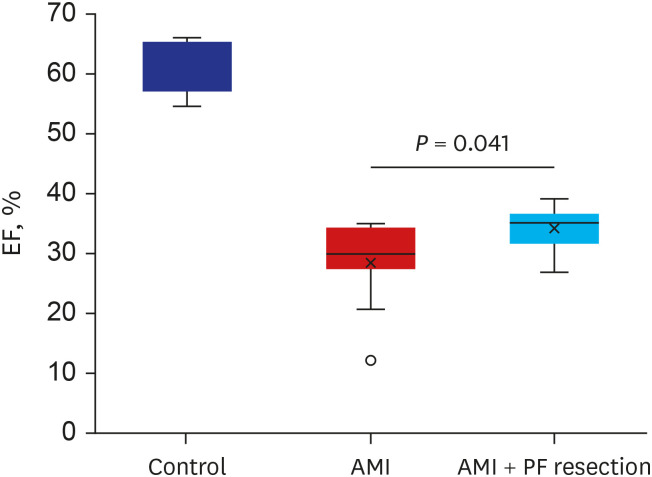
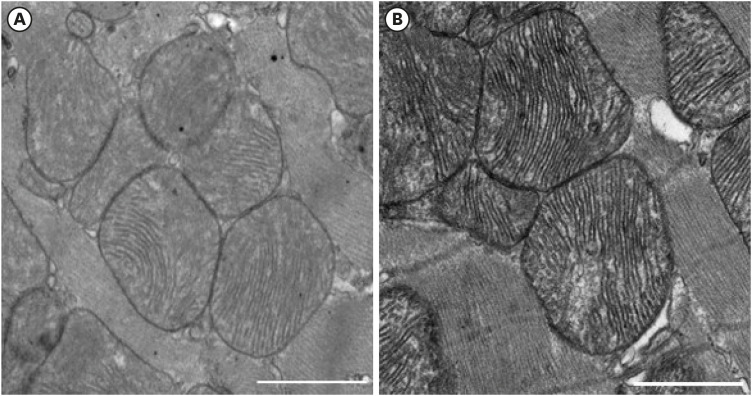
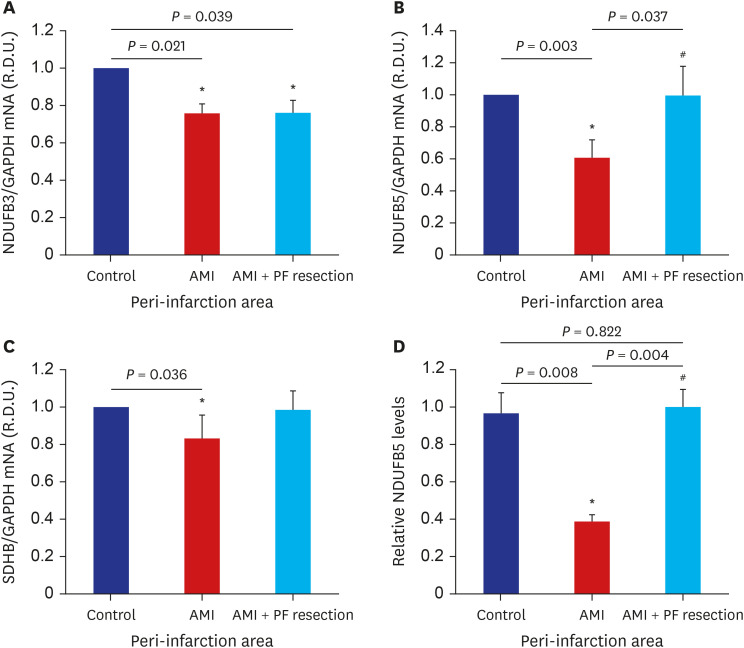
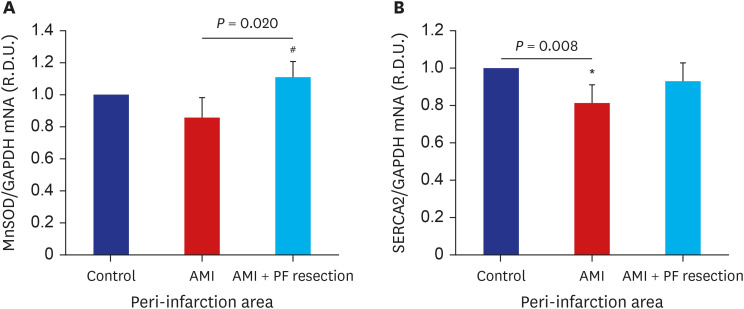
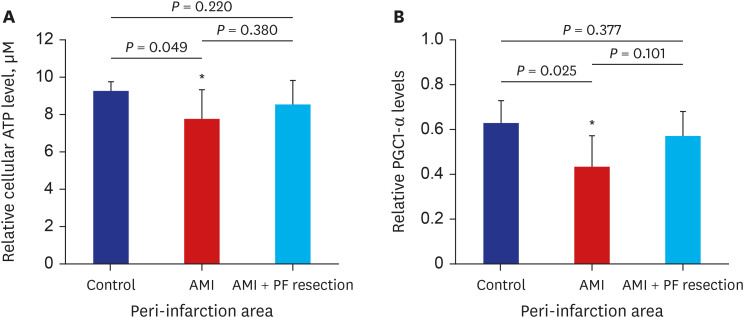
There was significant improvement of systolic function in AMI with PF resection compared with the AMI group in the echocardiogram. Even though the electron microscopic morphology for the mitochondria seems to be similar between the AMI with PF resection and AMI groups, there was an improved expression of PGC-1α and responsive OXPHOS including NDUFB3, NDUFB5 and SDHB are associated with the ATP levels in the AMI with PF resection compared with those in the AMI group. In addition, the expression levels of antioxidant enzymes (MnSOD) and SERCA2 were improved in the AMI with PF resection compared with those in the AMI group.
Conclusion
Surgical resection of PF might ameliorate myocardial mitochondria dysfunction in obese AMI.
Figure
Reference
-
1. Ruan Y, Guo Y, Zheng Y, Huang Z, Sun S, Kowal P, et al. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: results from SAGE Wave 1. BMC Public Health. 2018; 18(1):778. PMID: 29925336.
Article2. Song DK, Hong YS, Lee H, Oh JY, Sung YA, Kim Y. Increased epicardial adipose tissue thickness in type 2 diabetes mellitus and obesity. Diabetes Metab J. 2015; 39(5):405–413. PMID: 26566498.
Article3. Bentley-Lewis R, Adler GK, Perlstein T, Seely EW, Hopkins PN, Williams GH, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007; 92(11):4472–4475. PMID: 17726083.
Article4. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med. 2002; 347(5):305–313. PMID: 12151467.
Article5. Shin SY, Yong HS, Lim HE, Na JO, Choi CU, Choi JI, et al. Total and interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2011; 22(6):647–655. PMID: 21235672.
Article6. Bambace C, Sepe A, Zoico E, Telesca M, Olioso D, Venturi S, et al. Inflammatory profile in subcutaneous and epicardial adipose tissue in men with and without diabetes. Heart Vessels. 2014; 29(1):42–48. PMID: 23296264.
Article7. Naftali-Shani N, Levin-Kotler LP, Palevski D, Amit U, Kain D, Landa N, et al. Left ventricular dysfunction switches mesenchymal stromal cells toward an inflammatory phenotype and impairs their reparative properties via Toll-like receptor-4. Circulation. 2017; 135(23):2271–2287. PMID: 28356441.
Article8. Ruiz-Villalba A, Simón AM, Pogontke C, Castillo MI, Abizanda G, Pelacho B, et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. J Am Coll Cardiol. 2015; 65(19):2057–2066. PMID: 25975467.
Article9. McKenney-Drake ML, Rodenbeck SD, Bruning RS, Kole A, Yancey KW, Alloosh M, et al. Epicardial adipose tissue removal potentiates outward remodeling and arrests coronary atherogenesis. Ann Thorac Surg. 2017; 103(5):1622–1630. PMID: 28223054.
Article10. Chang HX, Zhao XJ, Zhu QL, Hou Q, Li Y. Removal of epicardial adipose tissue after myocardial infarction improves cardiac function. Herz. 2018; 43(3):258–264. PMID: 28378031.
Article11. Bière L, Behaghel V, Mateus V, Assunção A Jr, Gräni C, Ouerghi K, et al. Relation of quantity of subepicardial adipose tissue to infarct size in patients with ST-elevation myocardial infarction. Am J Cardiol. 2017; 119(12):1972–1978. PMID: 28438306.
Article12. Johannsen DL, Ravussin E. The role of mitochondria in health and disease. Curr Opin Pharmacol. 2009; 9(6):780–786. PMID: 19796990.
Article13. Kang KW, Kim OS, Chin JY, Kim WH, Park SH, Choi YJ, et al. Diastolic dysfunction induced by a high-fat diet is associated with mitochondrial abnormality and adenosine triphosphate levels in rats. Endocrinol Metab (Seoul). 2015; 30(4):557–568. PMID: 26790384.
Article14. Zhu N, Yan X, Li H, Wang H. Clinical significance of serum PGC-1 alpha levels in diabetes mellitus with myocardial infarction patients and reduced ROS-oxidative stress in diabetes mellitus with myocardial infarction model. Diabetes Metab Syndr Obes. 2020; 13:4041–4049. PMID: 33149643.15. Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L. PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat. 2016; 125:8–18. PMID: 27418542.
Article16. Wojtovich AP, Smith CO, Haynes CM, Nehrke KW, Brookes PS. Physiological consequences of complex II inhibition for aging, disease, and the mKATP channel. Biochim Biophys Acta. 2013; 1827(5):598–611. PMID: 23291191.
Article17. Matsubara LS, Matsubara BB, Okoshi MP, Cicogna AC, Janicki JS. Alterations in myocardial collagen content affect rat papillary muscle function. Am J Physiol Heart Circ Physiol. 2000; 279(4):H1534–H1539. PMID: 11009438.
Article18. Heaps CL, Tharp DL, Bowles DK. Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 2005; 288(2):H568–H576. PMID: 15458946.19. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978; 58(6):1072–1083. PMID: 709763.
Article20. Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005; 54(7):1926–1933. PMID: 15983191.
Article21. Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003; 34(3):267–273. PMID: 12808457.
Article22. Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Taguchi H, Yoshikawa J, et al. Epicardial adipose tissue accumulation is associated with renal dysfunction and coronary plaque morphology on multidetector computed tomography. Circ J. 2016; 80(1):196–201. PMID: 26497330.
Article23. Patel VB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes. 2016; 65(1):85–95. PMID: 26224885.
Article24. Packer M. Leptin-aldosterone-neprilysin axis: identification of its distinctive role in the pathogenesis of the three phenotypes of heart failure in people with obesity. Circulation. 2018; 137(15):1614–1631. PMID: 29632154.25. Patel VB, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev. 2017; 22(6):889–902. PMID: 28762019.
Article26. Wu CK, Tsai HY, Su MM, Wu YF, Hwang JJ, Lin JL, et al. Evolutional change in epicardial fat and its correlation with myocardial diffuse fibrosis in heart failure patients. J Clin Lipidol. 2017; 11(6):1421–1431. PMID: 29050981.
Article27. Iacobellis G, Leonetti F, Singh N, M Sharma A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007; 115(2):272–273. PMID: 16759715.
Article28. Parra V, Verdejo H, del Campo A, Pennanen C, Kuzmicic J, Iglewski M, et al. The complex interplay between mitochondrial dynamics and cardiac metabolism. J Bioenerg Biomembr. 2011; 43(1):47–51. PMID: 21258852.
Article29. Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007; 12(5):815–833. PMID: 17294078.
Article30. Javadov S, Karmazyn M. Mitochondrial permeability transition pore opening as an endpoint to initiate cell death and as a putative target for cardioprotection. Cell Physiol Biochem. 2007; 20(1-4):1–22. PMID: 17595511.
Article31. Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006; 49(6):1434–1446. PMID: 16612592.
Article32. Dong F, Zhang X, Yang X, Esberg LB, Yang H, Zhang Z, et al. Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J Endocrinol. 2006; 188(1):25–36. PMID: 16394172.
Article33. Ren J, Dong F, Cai GJ, Zhao P, Nunn JM, Wold LE, et al. Interaction between age and obesity on cardiomyocyte contractile function: role of leptin and stress signaling. PLoS One. 2010; 5(4):e10085. PMID: 20396382.
Article34. Dong F, Zhang X, Ren J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor-NADPH oxidase pathway. Hypertension. 2006; 47(2):222–229. PMID: 16380530.
Article35. Minhas KM, Khan SA, Raju SV, Phan AC, Gonzalez DR, Skaf MW, et al. Leptin repletion restores depressed beta-adrenergic contractility in ob/ob mice independently of cardiac hypertrophy. J Physiol. 2005; 565(Pt 2):463–474. PMID: 15760936.
Article36. Ren J, Sowers JR, Walsh MF, Brown RA. Reduced contractile response to insulin and IGF-I in ventricular myocytes from genetically obese Zucker rats. Am J Physiol Heart Circ Physiol. 2000; 279(4):H1708–H1714. PMID: 11009458.
Article37. Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc Res. 2010; 88(2):229–240. PMID: 20639213.
Article38. Bers DM, Shannon TR. Calcium movements inside the sarcoplasmic reticulum of cardiac myocytes. J Mol Cell Cardiol. 2013; 58:59–66. PMID: 23321551.
Article39. Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006; 116(3):615–622. PMID: 16511594.
Article40. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114(12):1752–1761. PMID: 15599400.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Pericardial Effusion after Acute Myocardial Infarction
- Invasive Treatment of Acute Myocardial Infarction: What is the Optimal Therapy for Acute Myocardial Infarction?
- Peiminine inhibits myocardial injury and fibrosis after myocardial infarction in rats by regulating mitogen-activated protein kinase pathway
- A Case of Early Developed Left Ventricular Free Wall Rupture Followed by Acute Inferior Myocardial Infarction
- Myocardial fractional flow reserve in acute myocardial infarction