Int J Stem Cells.
2022 Feb;15(1):104-111. 10.15283/ijsc21209.
Generation of Highly Expandable Intestinal Spheroids Composed of Stem Cells
- Affiliations
-
- 1Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea
- 2KRIBB School of Bioscience, Korea University of Science and Technology, Daejeon, Korea
- KMID: 2526742
- DOI: http://doi.org/10.15283/ijsc21209
Abstract
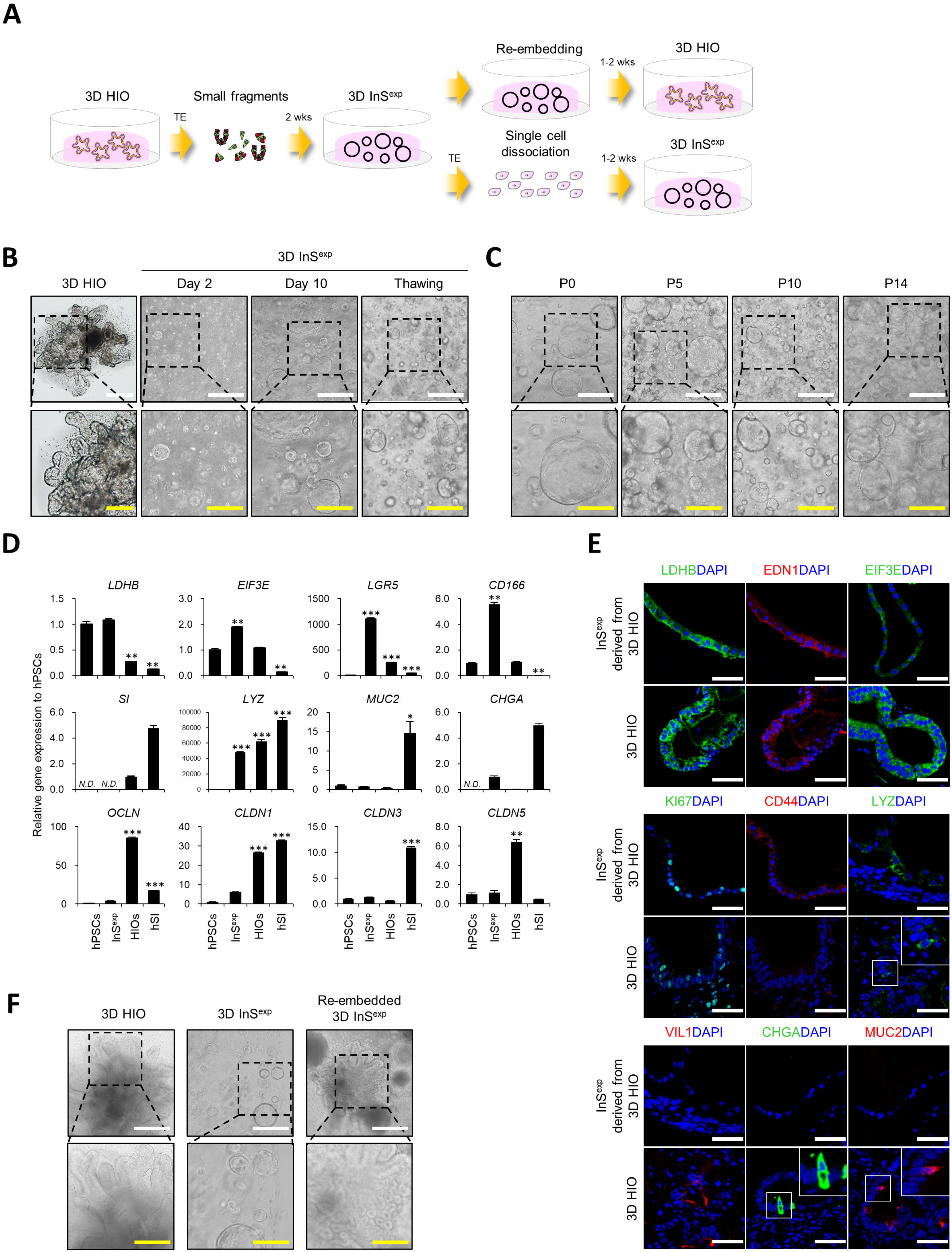
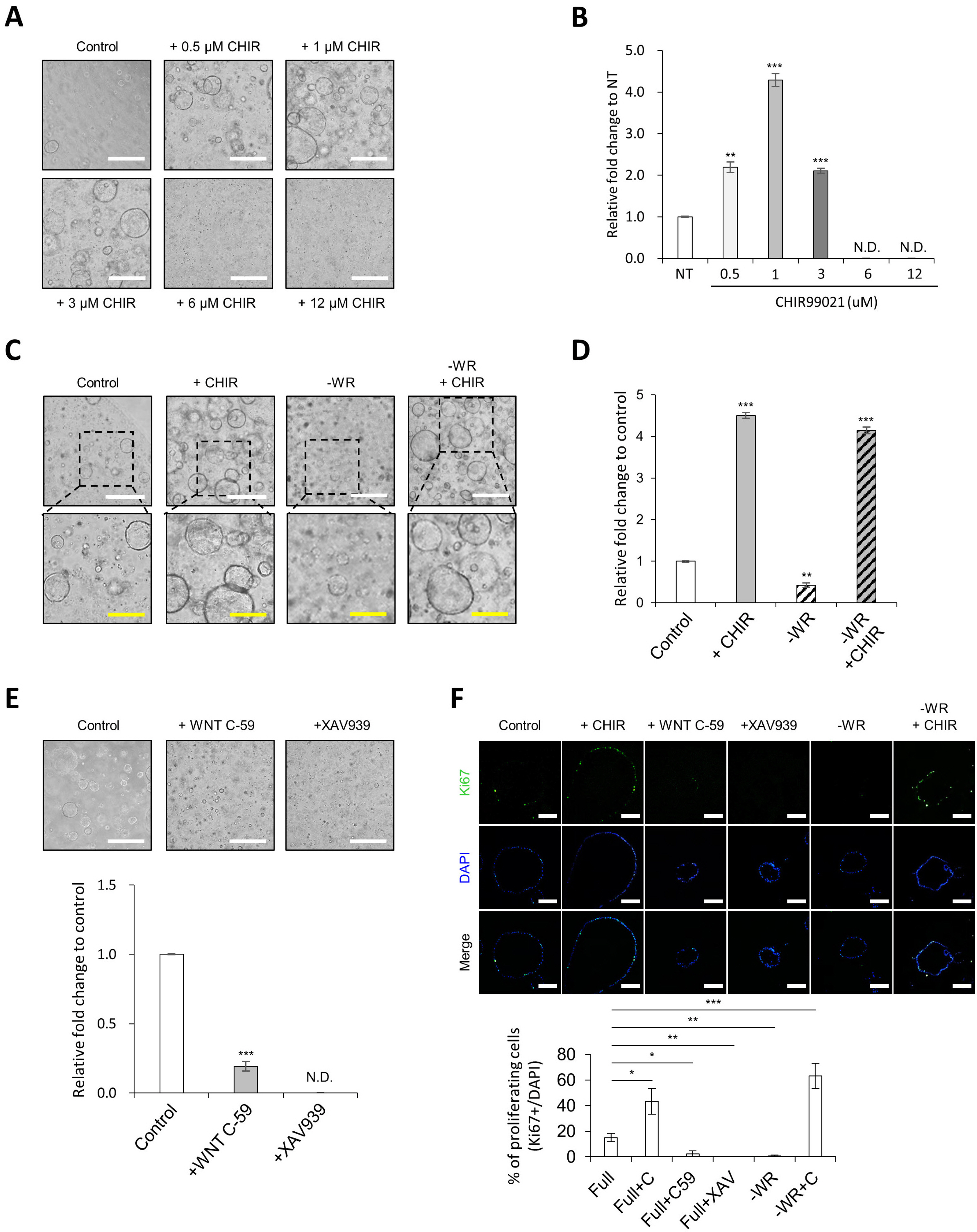
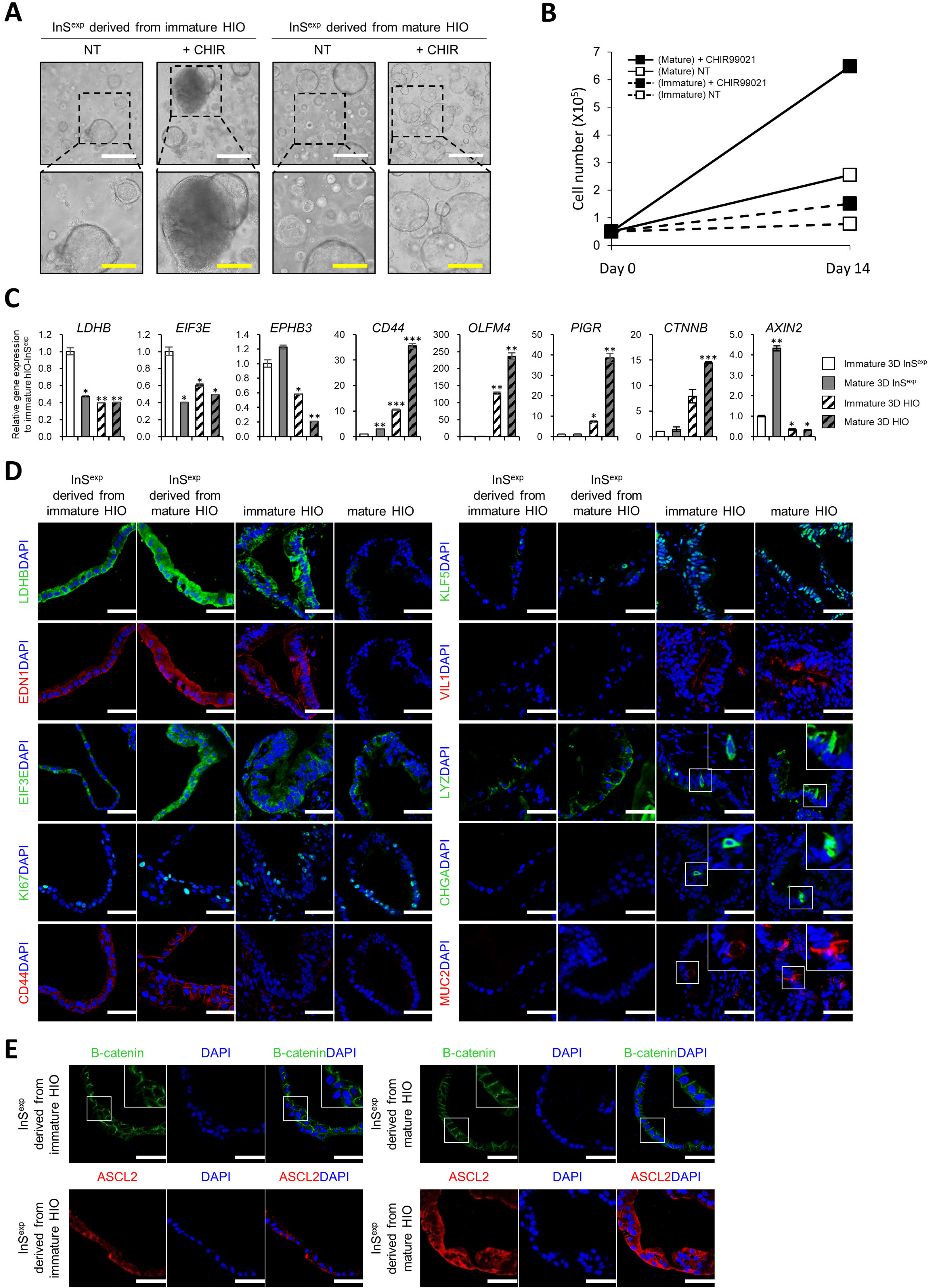
- Many of early findings regarding intestinal stem cells (ISCs) and their niche in the human intestine have relied on colorectal cancer cell lines and labor-intensive and time-consuming mouse models. However, these models cannot accurately recapitulate the physiologically relevant aspects of human ISCs. In this study, we demonstrate a reliable and robust culture method for 3D expanding intestinal spheroids (InSexp ) mainly comprising ISCs and progenitors, which can be derived from 3D human intestinal organoids (HIOs). We did functional chararcterization of InSexp derived from 3D HIOs, differentiated from human pluripotent stem cells, and optimization culture methods. Our results indicate that InSexp can be rapidly expanded and easily passaged, and show enhanced growth rates via WNT pathway activation. InSexp are capable of exponential cell expansion and cryopreservation. Furthermore, in vitro-matured HIO-derived InSexp proliferate faster than immature HIO-derived InSexp with preservation of the parental HIO characteristics. These findings may facilitate the development of scalable culture systems for the long-term maintenance of human ISCs and provide an alternative platform for studying ISC biology.
Keyword
Figure
Cited by 2 articles
-
Lo and Behold, the Lab-Grown Organs Have Arrived!
Jaesang Kim
Int J Stem Cells. 2022;15(1):1-2. doi: 10.15283/ijsc22026.Peripheral Neuron-Organoid Interaction Induces Colonic Epithelial Differentiation via Non-Synaptic Substance P Secretion
Young Hyun Che, In Young Choi, Chan Eui Song, Chulsoon Park, Seung Kwon Lim, Jeong Hee Kim, Su Haeng Sung, Jae Hoon Park, Sun Lee, Yong Jun Kim
Int J Stem Cells. 2023;16(3):269-280. doi: 10.15283/ijsc23026.
Reference
-
References
1. Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. 2011; Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 470:105–109. DOI: 10.1038/nature09691. PMID: 21151107. PMCID: PMC3033971.
Article2. Jung KB, Lee H, Son YS, Lee MO, Kim YD, Oh SJ, Kwon O, Cho S, Cho HS, Kim DS, Oh JH, Zilbauer M, Min JK, Jung CR, Kim J, Son MY. 2018; Interleukin-2 induces the in vitro maturation of human pluripotent stem cell-derived intestinal organoids. Nat Commun. 9:3039. DOI: 10.1038/s41467-018-05450-8. PMID: 30072687. PMCID: PMC6072745. PMID: a475745ef0d7496ba465e8488bf0de6b.
Article3. Dedhia PH, Bertaux-Skeirik N, Zavros Y, Spence JR. 2016; Organoid models of human gastrointestinal development and disease. Gastroenterology. 150:1098–1112. DOI: 10.1053/j.gastro.2015.12.042. PMID: 26774180. PMCID: PMC4842135.
Article4. Kim J, Koo BK, Knoblich JA. 2020; Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 21:571–584. DOI: 10.1038/s41580-020-0259-3. PMID: 32636524. PMCID: PMC7339799.
Article5. Gracz AD, Magness ST. 2014; Defining hierarchies of stemness in the intestine: evidence from biomarkers and regulatory pathways. Am J Physiol Gastrointest Liver Physiol. 307:G260–G273. DOI: 10.1152/ajpgi.00066.2014. PMID: 24924746. PMCID: PMC4121637.
Article6. Son MY, Sim H, Son YS, Jung KB, Lee MO, Oh JH, Chung SK, Jung CR, Kim J. 2017; Distinctive genomic signature of neural and intestinal organoids from familial Parkinson's disease patient-derived induced pluripotent stem cells. Neuropathol Appl Neurobiol. 43:584–603. DOI: 10.1111/nan.12396. PMID: 28235153.
Article7. Kwon O, Jung KB, Lee KR, Son YS, Lee H, Kim JJ, Kim K, Lee S, Song YK, Jung J, Park K, Kim DS, Son MJ, Lee MO, Han TS, Cho HS, Oh SJ, Chung H, Kim SH, Chung KS, Kim J, Jung CR, Son MY. 2021; The development of a functional human small intestinal epithelium model for drug absorption. Sci Adv. 7:eabh1586. DOI: 10.1126/sciadv.abh1586. PMID: 34078609.
Article8. Lee H, Son YS, Lee MO, Ryu JW, Park K, Kwon O, Jung KB, Kim K, Ryu TY, Baek A, Kim J, Jung CR, Ryu CM, Park YJ, Han TS, Kim DS, Cho HS, Son MY. 2020; Low-dose interleukin-2 alleviates dextran sodium sulfate-induced colitis in mice by recovering intestinal integrity and inhibiting AKT-dependent pathways. Theranostics. 10:5048–5063. DOI: 10.7150/thno.41534. PMID: 32308767. PMCID: PMC7163458.
Article9. Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. 2014; Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods. 11:106–112. DOI: 10.1038/nmeth.2737. PMID: 24292484. PMCID: PMC3951815.
Article10. Fujii M, Matano M, Nanki K, Sato T. 2015; Efficient genetic engineering of human intestinal organoids using electroporation. Nat Protoc. 10:1474–1485. Erratum in: Nat Protoc 2019; 14:2595. DOI: 10.1038/nprot.2015.088. PMID: 26334867.
Article11. Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, Ootani A, Roelf K, Lee M, Yuan J, Li X, Bolen CR, Wilhelmy J, Davies PS, Ueno H, von Furstenberg RJ, Belgrader P, Ziraldo SB, Ordonez H, Henning SJ, Wong MH, Snyder MP, Weissman IL, Hsueh AJ, Mikkelsen TS, Garcia KC, Kuo CJ. 2017; Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature. 545:238–242. DOI: 10.1038/nature22313. PMID: 28467820. PMCID: PMC5641471.
Article12. Santos AJM, Lo YH, Mah AT, Kuo CJ. 2018; The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol. 28:1062–1078. DOI: 10.1016/j.tcb.2018.08.001. PMID: 30195922. PMCID: PMC6338454.
Article13. Costa J, Ahluwalia A. 2019; Advances and current challenges in intestinal in vitro model engineering: a digest. Front Bioeng Biotechnol. 7:144. DOI: 10.3389/fbioe.2019.00144. PMID: 31275931. PMCID: PMC6591368.
Article14. Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, Smith NR, Lei NY, Davies PS, Fuller MK, Haug JS, McClain M, Gracz AD, Ding S, Stelzner M, Dunn JC, Magness ST, Wong MH, Martin MG, Helmrath M, Li L. 2013; Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology. 145:383–395.e1-e21. DOI: 10.1053/j.gastro.2013.04.050. PMID: 23644405. PMCID: PMC3781924.
Article15. Watson CL, Mahe MM, Múnera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, Grabowski G, Finkbeiner SR, Spence JR, Shroyer NF, Wells JM, Helmrath MA. 2014; An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 20:1310–1314. DOI: 10.1038/nm.3737. PMID: 25326803. PMCID: PMC4408376.
Article16. Poling HM, Wu D, Brown N, Baker M, Hausfeld TA, Huynh N, Chaffron S, Dunn JCY, Hogan SP, Wells JM, Helmrath MA, Mahe MM. 2018; Mechanically induced development and maturation of human intestinal organoids in vivo. Nat Biomed Eng. 2:429–442. DOI: 10.1038/s41551-018-0243-9. PMID: 30151330. PMCID: PMC6108544.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two-Cell Spheroid Angiogenesis Assay System Using Both Endothelial Colony Forming Cells and Mesenchymal Stem Cells
- Generation of Cortical Brain Organoid with Vascularization by Assembling with Vascular Spheroid
- Development of Hetero-Cell Type Spheroids Via Core–Shell Strategy for Enhanced Wound Healing Effect of Human AdiposeDerived Stem Cells
- Mesenchymal Stem Cell Spheroids: A Promising Tool for Vascularized Tissue Regeneration
- Navigating the Landscape of Intestinal Regeneration: A Spotlight on Quiescence Regulation and Fetal Reprogramming