Korean J Physiol Pharmacol.
2022 Mar;26(2):69-75. 10.4196/kjpp.2022.26.2.69.
Altered synaptic connections and inhibitory network of the primary somatosensory cortex in chronic pain
- Affiliations
-
- 1Departments of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea.
- 2Departments of Biomedical Sciences, Seoul National University College of Medicine, Seoul 03080, Korea
- 3Neuroscience Research Institute, Seoul National University College of Medicine, Seoul 03080, Korea
- KMID: 2526726
- DOI: http://doi.org/10.4196/kjpp.2022.26.2.69
Abstract
- Chronic pain is induced by tissue or nerve damage and is accompanied by pain hypersensitivity (i.e., allodynia and hyperalgesia). Previous studies using in vivo two-photon microscopy have shown functional and structural changes in the primary somatosensory (S1) cortex at the cellular and synaptic levels in inflammatory and neuropathic chronic pain. Furthermore, alterations in local cortical circuits were revealed during the development of chronic pain. In this review, we summarize recent findings regarding functional and structural plastic changes of the S1 cortex and alteration of the S1 inhibitory network in chronic pain. Finally, we discuss potential neuromodulators driving modified cortical circuits and suggest further studies to understand the cortical mechanisms that induce pain hypersensitivity.
Keyword
Figure
Reference
-
1. Woolf CJ. American College of Physicians. American Physiological Society. 2004; Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 140:441–451. DOI: 10.7326/0003-4819-140-8-200404200-00010. PMID: 15023710.
Article2. Baron R. 2006; Mechanisms of disease: neuropathic pain--a clinical perspective. Nat Clin Pract Neurol. 2:95–106. DOI: 10.1038/ncpneuro0113. PMID: 16932531.
Article3. Latremoliere A, Woolf CJ. 2009; Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 10:895–926. DOI: 10.1016/j.jpain.2009.06.012. PMID: 19712899. PMCID: PMC2750819.
Article4. Costigan M, Scholz J, Woolf CJ. 2009; Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 32:1–32. DOI: 10.1146/annurev.neuro.051508.135531. PMID: 19400724. PMCID: PMC2768555.
Article5. Woolf CJ, Shortland P, Coggeshall RE. 1992; Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 355:75–78. DOI: 10.1038/355075a0. PMID: 1370574.
Article6. Woodbury CJ, Kullmann FA, McIlwrath SL, Koerber HR. 2008; Identity of myelinated cutaneous sensory neurons projecting to nocireceptive laminae following nerve injury in adult mice. J Comp Neurol. 508:500–509. DOI: 10.1002/cne.21693. PMID: 18335545. PMCID: PMC2664515.
Article7. Kohno T, Moore KA, Baba H, Woolf CJ. 2003; Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol. 548(Pt 1):131–138. DOI: 10.1113/jphysiol.2002.036186. PMID: 12576493. PMCID: PMC2342789.
Article8. Baba H, Doubell TP, Woolf CJ. 1999; Peripheral inflammation facilitates Abeta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci. 19:859–867. DOI: 10.1523/JNEUROSCI.19-02-00859.1999. PMID: 9880605. PMCID: PMC6782212.
Article9. Gwak YS, Hulsebosch CE. 2011; Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury. Curr Pain Headache Rep. 15:215–222. DOI: 10.1007/s11916-011-0186-2. PMID: 21387163.
Article10. Blom SM, Pfister JP, Santello M, Senn W, Nevian T. 2014; Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J Neurosci. 34:5754–5764. DOI: 10.1523/JNEUROSCI.3667-13.2014. PMID: 24760836. PMCID: PMC6608297.
Article11. Seifert F, Maihöfner C. 2009; Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 66:375–390. DOI: 10.1007/s00018-008-8428-0. PMID: 18791842.
Article12. Bak MS, Park H, Kim SK. 2021; Neural plasticity in the brain during neuropathic pain. Biomedicines. 9:624. DOI: 10.3390/biomedicines9060624. PMID: 34072638. PMCID: PMC8228570. PMID: 8ec93ead3216442faa82c91fbedef1f0.
Article13. Kim CE, Kim YK, Chung G, Jeong JM, Lee DS, Kim J, Kim SJ. 2014; Large-scale plastic changes of the brain network in an animal model of neuropathic pain. Neuroimage. 98:203–215. DOI: 10.1016/j.neuroimage.2014.04.063. PMID: 24799136.
Article14. Eto K, Ishibashi H, Yoshimura T, Watanabe M, Miyamoto A, Ikenaka K, Moorhouse AJ, Nabekura J. 2012; Enhanced GABAergic activity in the mouse primary somatosensory cortex is insufficient to alleviate chronic pain behavior with reduced expression of neuronal potassium-chloride cotransporter. J Neurosci. 32:16552–16559. DOI: 10.1523/JNEUROSCI.2104-12.2012. PMID: 23175811. PMCID: PMC6621771.
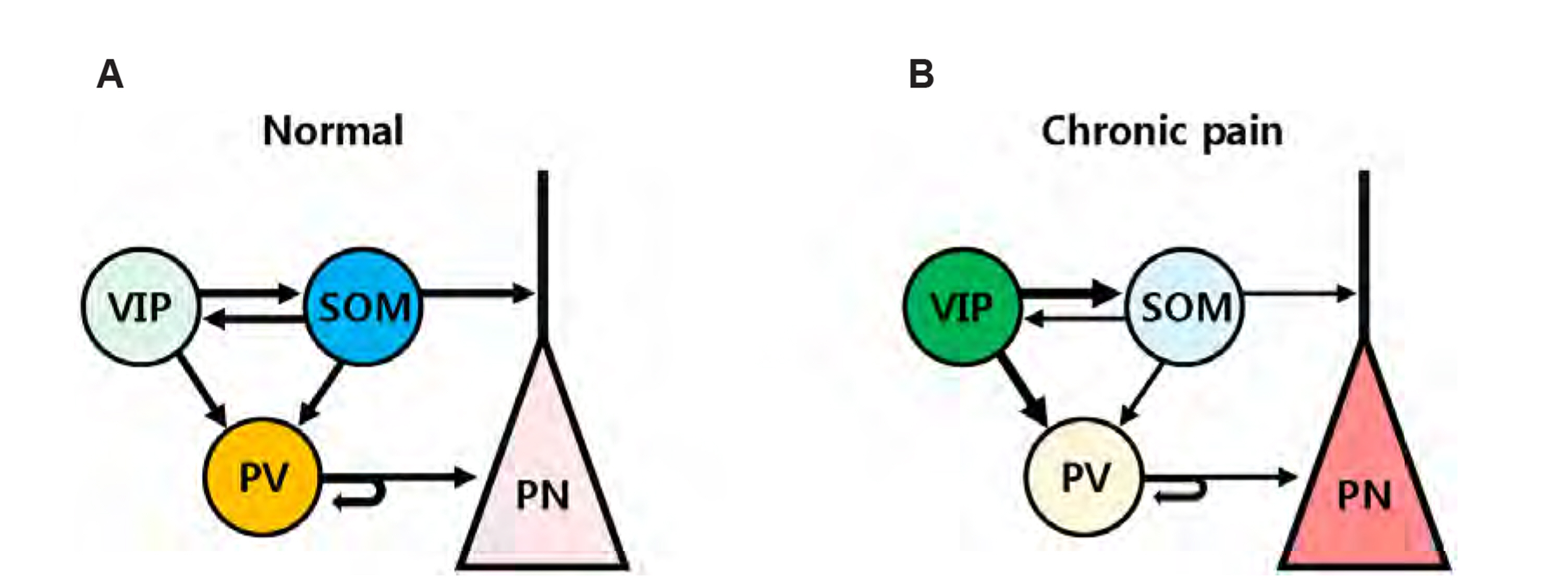
Article15. Cichon J, Blanck TJJ, Gan WB, Yang G. 2017; Activation of cortical somatostatin interneurons prevents the development of neuropathic pain. Nat Neurosci. 20:1122–1132. DOI: 10.1038/nn.4595. PMID: 28671692. PMCID: PMC5559271.
Article16. Wei JA, Hu X, Zhang B, Liu L, Chen K, So KF, Li M, Zhang L. 2021; Electroacupuncture activates inhibitory neural circuits in the somatosensory cortex to relieve neuropathic pain. iScience. 24:102066. DOI: 10.1016/j.isci.2021.102066. PMID: 33554069. PMCID: PMC7859294.
Article17. Kim SK, Kato G, Ishikawa T, Nabekura J. 2011; Phase-specific plasticity of synaptic structures in the somatosensory cortex of living mice during neuropathic pain. Mol Pain. 7:87. DOI: 10.1186/1744-8069-7-87. PMID: 22067412. PMCID: PMC3223139.
Article18. Kim SK, Nabekura J. 2011; Rapid synaptic remodeling in the adult somatosensory cortex following peripheral nerve injury and its association with neuropathic pain. J Neurosci. 31:5477–5482. DOI: 10.1523/JNEUROSCI.0328-11.2011. PMID: 21471384. PMCID: PMC6622722.
Article19. Kim SK, Hayashi H, Ishikawa T, Shibata K, Shigetomi E, Shinozaki Y, Inada H, Roh SE, Kim SJ, Lee G, Bae H, Moorhouse AJ, Mikoshiba K, Fukazawa Y, Koizumi S, Nabekura J. 2016; Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Invest. 126:1983–1997. DOI: 10.1172/JCI82859. PMID: 27064281. PMCID: PMC4855913.
Article20. DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Anderson S, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, González-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, et al. 2013; New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 14:202–216. DOI: 10.1038/nrn3444. PMID: 23385869. PMCID: PMC3619199.
Article21. Cichon J, Gan WB. 2015; Branch-specific dendritic Ca2+ spikes cause persistent synaptic plasticity. Nature. 520:180–185. DOI: 10.1038/nature14251. PMID: 25822789. PMCID: PMC4476301.
Article22. Beierlein M, Gibson JR, Connors BW. 2003; Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 90:2987–3000. DOI: 10.1152/jn.00283.2003. PMID: 12815025.
Article23. Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. 2013; Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 16:1068–1076. DOI: 10.1038/nn.3446. PMID: 23817549. PMCID: PMC3729586.
Article24. Cha MH, Kim DS, Cho ZH, Sohn JH, Chung MA, Lee HJ, Nam TS, Lee BH. 2009; Modification of cortical excitability in neuropathic rats: a voltage-sensitive dye study. Neurosci Lett. 464:117–121. DOI: 10.1016/j.neulet.2009.08.024. PMID: 19682547.
Article25. Endo T, Spenger C, Hao J, Tominaga T, Wiesenfeld-Hallin Z, Olson L, Xu XJ. 2008; Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain. 138:292–300. DOI: 10.1016/j.pain.2007.12.017. PMID: 18258366.
Article26. Xiong W, Ping X, Ripsch MS, Chavez GSC, Hannon HE, Jiang K, Bao C, Jadhav V, Chen L, Chai Z, Ma C, Wu H, Feng J, Blesch A, White FA, Jin X. 2017; Enhancing excitatory activity of somatosensory cortex alleviates neuropathic pain through regulating homeostatic plasticity. Sci Rep. 7:12743. DOI: 10.1038/s41598-017-12972-6. PMID: 28986567. PMCID: PMC5630599.
Article27. Okada T, Kato D, Nomura Y, Obata N, Quan X, Morinaga A, Yano H, Guo Z, Aoyama Y, Tachibana Y, Moorhouse AJ, Matoba O, Takiguchi T, Mizobuchi S, Wake H. 2021; Pain induces stable, active microcircuits in the somatosensory cortex that provide a therapeutic target. Sci Adv. 7:eabd8261. DOI: 10.1126/sciadv.abd8261. PMID: 33741588. PMCID: PMC7978434.
Article28. Eto K, Wake H, Watanabe M, Ishibashi H, Noda M, Yanagawa Y, Nabekura J. 2011; Inter-regional contribution of enhanced activity of the primary somatosensory cortex to the anterior cingulate cortex accelerates chronic pain behavior. J Neurosci. 31:7631–7636. DOI: 10.1523/JNEUROSCI.0946-11.2011. PMID: 21613476. PMCID: PMC6633125.
Article29. Jones AF, Sheets PL. 2020; Sex-specific disruption of distinct mPFC inhibitory neurons in spared-nerve injury model of neuropathic pain. Cell Rep. 31:107729. DOI: 10.1016/j.celrep.2020.107729. PMID: 32521254. PMCID: PMC7372908.
Article30. Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. 2003; Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 424:938–942. DOI: 10.1038/nature01868. PMID: 12931188.
Article31. Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Möhler H, Zeilhofer HU. 2008; Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 451:330–334. DOI: 10.1038/nature06493. PMID: 18202657.
Article32. Harding EK, Salter MW. 2017; VIP cortical conductors set the tone for chronic pain. Nat Neurosci. 20:1037–1038. DOI: 10.1038/nn.4609. PMID: 28745719.
Article33. Pakan JM, Lowe SC, Dylda E, Keemink SW, Currie SP, Coutts CA, Rochefort NL. 2016; Behavioral-state modulation of inhibition is context-dependent and cell type specific in mouse visual cortex. Elife. 5:e14985. DOI: 10.7554/eLife.14985. PMID: 27552056. PMCID: PMC5030095. PMID: 88275e5caafb44cea3b718e0e49e33d5.
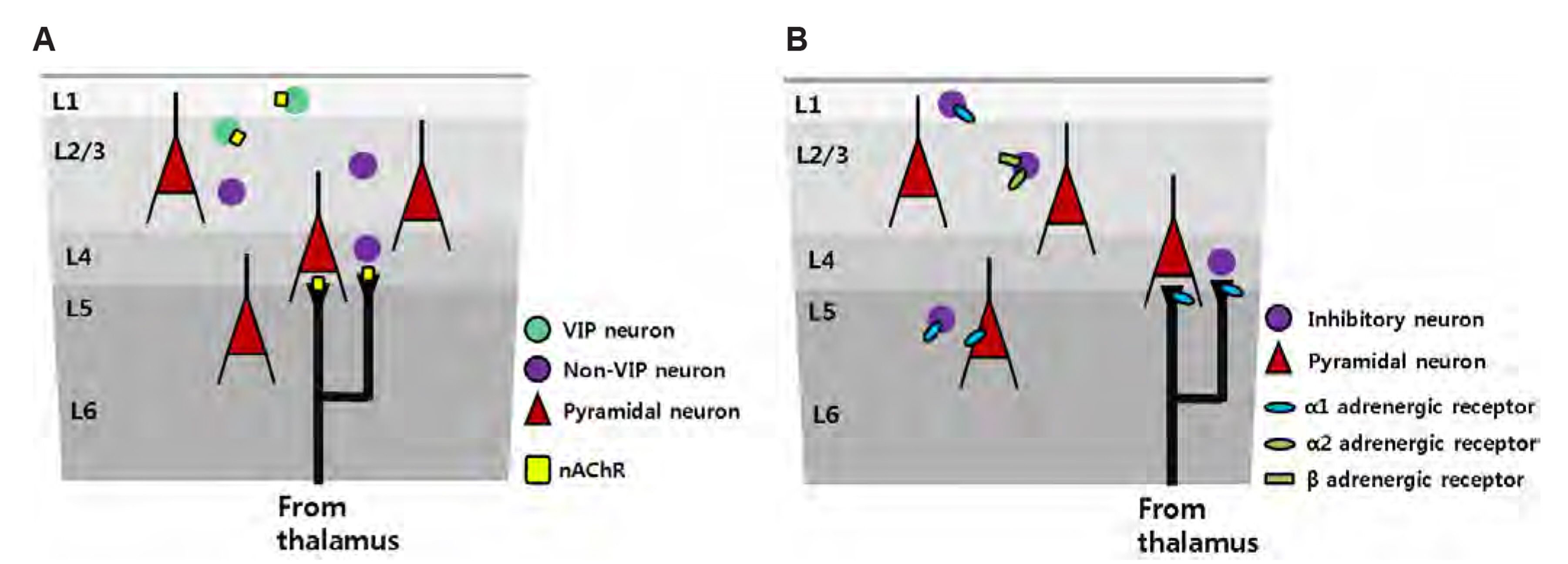
Article34. Gasselin C, Hohl B, Vernet A, Crochet S, Petersen CCH. 2021; Cell-type-specific nicotinic input disinhibits mouse barrel cortex during active sensing. Neuron. 109:778–787.e3. DOI: 10.1016/j.neuron.2020.12.018. PMID: 33472037. PMCID: PMC7927912.
Article35. Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. 2013; Cortical interneurons that specialize in disinhibitory control. Nature. 503:521–524. DOI: 10.1038/nature12676. PMID: 24097352. PMCID: PMC4017628.
Article36. Poorthuis RB, Enke L, Letzkus JJ. 2014; Cholinergic circuit modulation through differential recruitment of neocortical interneuron types during behaviour. J Physiol. 592:4155–4164. DOI: 10.1113/jphysiol.2014.273862. PMID: 24879871. PMCID: PMC4215768.
Article37. Arroyo S, Bennett C, Aziz D, Brown SP, Hestrin S. 2012; Prolonged disynaptic inhibition in the cortex mediated by slow, non-α7 nicotinic excitation of a specific subset of cortical interneurons. J Neurosci. 32:3859–3864. DOI: 10.1523/JNEUROSCI.0115-12.2012. PMID: 22423106. PMCID: PMC3320796.
Article38. Hedrick T, Waters J. 2015; Acetylcholine excites neocortical pyramidal neurons via nicotinic receptors. J Neurophysiol. 113:2195–2209. DOI: 10.1152/jn.00716.2014. PMID: 25589590. PMCID: PMC4416587.
Article39. Brombas A, Fletcher LN, Williams SR. 2014; Activity-dependent modulation of layer 1 inhibitory neocortical circuits by acetylcholine. J Neurosci. 34:1932–1941. DOI: 10.1523/JNEUROSCI.4470-13.2014. PMID: 24478372. PMCID: PMC6827591.
Article40. Donoghue JP, Carroll KL. 1987; Cholinergic modulation of sensory responses in rat primary somatic sensory cortex. Brain Res. 408:367–371. DOI: 10.1016/0006-8993(87)90407-0. PMID: 3594226.
Article41. Morrison JH, Foote SL. 1986; Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol. 243:117–138. DOI: 10.1002/cne.902430110. PMID: 3950077.
Article42. McBurney-Lin J, Lu J, Zuo Y, Yang H. 2019; Locus coeruleus-norepinephrine modulation of sensory processing and perception: a focused review. Neurosci Biobehav Rev. 105:190–199. DOI: 10.1016/j.neubiorev.2019.06.009. PMID: 31260703. PMCID: PMC6742544.
Article43. Sara SJ, Bouret S. 2012; Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 76:130–141. DOI: 10.1016/j.neuron.2012.09.011. PMID: 23040811.
Article44. Yoshimura M, Furue H. 2017; In vivo electrophysiological analysis of mechanisms of monoaminergic pain inhibitory systems. Pain. 158 Suppl 1:S85–S91. DOI: 10.1097/j.pain.0000000000000844. PMID: 28240646.
Article45. North RA, Yoshimura M. 1984; The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 349:43–55. DOI: 10.1113/jphysiol.1984.sp015141. PMID: 6145790. PMCID: PMC1199322.
Article46. Alba-Delgado C, Mico JA, Berrocoso E. 2021; Neuropathic pain increases spontaneous and noxious-evoked activity of locus coeruleus neurons. Prog Neuropsychopharmacol Biol Psychiatry. 105:110121. DOI: 10.1016/j.pnpbp.2020.110121. PMID: 33007320.
Article47. Brightwell JJ, Taylor BK. 2009; Noradrenergic neurons in the locus coeruleus contribute to neuropathic pain. Neuroscience. 160:174–185. DOI: 10.1016/j.neuroscience.2009.02.023. PMID: 19223010. PMCID: PMC2677992.
Article48. Alba-Delgado C, Llorca-Torralba M, Horrillo I, Ortega JE, Mico JA, Sánchez-Blázquez P, Meana JJ, Berrocoso E. 2013; Chronic pain leads to concomitant noradrenergic impairment and mood disorders. Biol Psychiatry. 73:54–62. DOI: 10.1016/j.biopsych.2012.06.033. PMID: 22854119.
Article49. Waterhouse BD, Mouradian R, Sessler FM, Lin RC. 2000; Differential modulatory effects of norepinephrine on synaptically driven responses of layer V barrel field cortical neurons. Brain Res. 868:39–47. DOI: 10.1016/S0006-8993(00)02261-7. PMID: 10841886.
Article50. Devilbiss DM, Waterhouse BD. 2000; Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse. 37:273–282. DOI: 10.1002/1098-2396(20000915)37:4<273::AID-SYN4>3.0.CO;2-#. PMID: 10891864.
Article51. Deitcher Y, Leibner Y, Kutzkel S, Zylbermann N, London M. 2019; Nonlinear relationship between multimodal adrenergic responses and local dendritic activity in primary sensory cortices. BioRxiv. 814657 [Preprint]. Available from: https://doi.org/10.1101/814657.cited 2021 Dec 5. DOI: 10.1101/814657.
Article52. Salgado H, Garcia-Oscos F, Patel A, Martinolich L, Nichols JA, Dinh L, Roychowdhury S, Tseng KY, Atzori M. 2011; Layer-specific noradrenergic modulation of inhibition in cortical layer II/III. Cereb Cortex. 21:212–221. DOI: 10.1093/cercor/bhq081. PMID: 20466749. PMCID: PMC3000571.
Article53. Lamour Y, Willer JC, Guilbaud G. 1983; Rat somatosensory (SmI) cortex: I. Characteristics of neuronal responses to noxious stimulation and comparison with responses to non-noxious stimulation. Exp Brain Res. 49:35–45. DOI: 10.1007/BF00235539. PMID: 6861935.
Article54. Chung JM, Surmeier DJ, Lee KH, Sorkin LS, Honda CN, Tsong Y, Willis WD. 1986; Classification of primate spinothalamic and somatosensory thalamic neurons based on cluster analysis. J Neurophysiol. 56:308–327. DOI: 10.1152/jn.1986.56.2.308. PMID: 3760923.
Article55. Kim YR, Kim CE, Yoon H, Kim SK, Kim SJ. 2019; S1 Employs feature-dependent differential selectivity of single cells and distributed patterns of populations to encode mechanosensations. Front Cell Neurosci. 13:132. DOI: 10.3389/fncel.2019.00132. PMID: 31024261. PMCID: PMC6460949.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neural circuit remodeling and structural plasticity in the cortex during chronic pain
- Neuroplasticity in chronic pain: insights into diagnosis and treatment
- Pulse-train Stimulation of Primary Somatosensory Cortex Blocks Pain Perception in Tail Clip Test
- A Case of Hemichorea with Primary Somatosensory Cortical Infarction
- Compensatory Hyperactivity of the Ipsilesional Red Nucleus in a Patient With Somatosensory Cortex Damage: A Case Report