J Korean Med Sci.
2022 Feb;37(7):e59. 10.3346/jkms.2022.37.e59.
Risk Stratification of Childhood Medulloblastoma Using Integrated Diagnosis: Discrepancies With Clinical Risk Stratification
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2526520
- DOI: http://doi.org/10.3346/jkms.2022.37.e59
Abstract
- Background
Recent genomic studies identified four discrete molecular subgroups of medulloblastoma (MB), and the risk stratification of childhood MB in the context of subgroups was refined in 2015. In this study, we investigated the effect of molecular subgroups on the risk stratification of childhood MB.
Methods
The nCounter® system and a customized cancer panel were used for molecular subgrouping and risk stratification in archived tissues.
Results
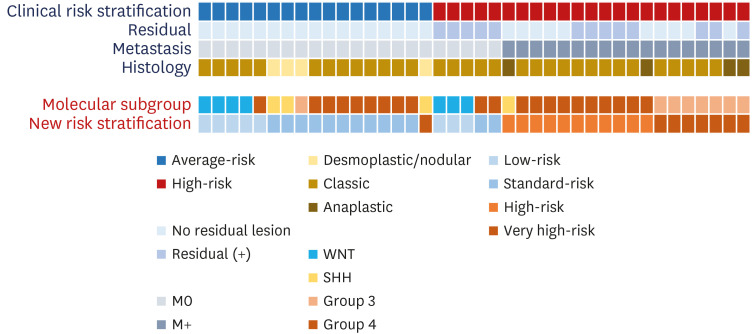
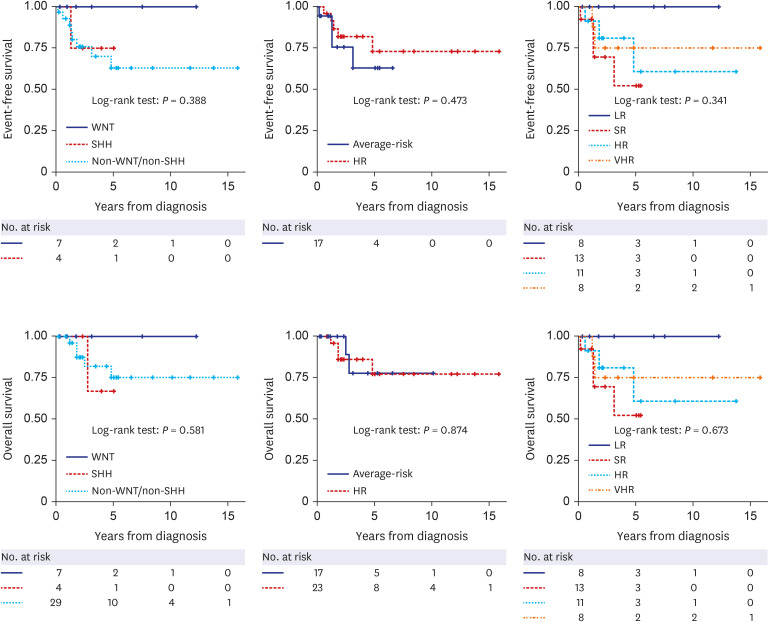
A total of 44 patients were included in this study. In clinical risk stratification, based on the presence of residual tumor/metastasis and histological findings, 24 and 20 patients were classified into the average-risk and high-risk groups, respectively. Molecular subgroups were successfully defined in 37 patients using limited gene expression analysis, and DNA panel sequencing additionally classified the molecular subgroups in three patients. Collectively, 40 patients were classified into molecular subgroups as follows: WNT (n = 7), SHH (n = 4), Group 3 (n = 8), and Group 4 (n = 21). Excluding the four patients whose molecular subgroups could not be determined, among the 17 average-risk group patients in clinical risk stratification, one patient in the SHH group with the TP53 variant was reclassified as very-high-risk using the new risk classification system. In addition, 5 of 23 patients who were initially classified as high-risk group in clinical risk stratification were reclassified into the low- or standard-risk groups in the new risk classification system.
Conclusion
The new risk stratification incorporating integrated diagnosis showed some discrepancies with clinical risk stratification. Risk stratification based on precise molecular subgrouping is needed for the tailored treatment of MB patients.
Figure
Reference
-
1. Northcott PA, Robinson GW, Kratz CP, Mabbott DJ, Pomeroy SL, Clifford SC, et al. Medulloblastoma. Nat Rev Dis Primers. 2019; 5(1):11. PMID: 30765705.
Article2. Thompson MC, Fuller C, Hogg TL, Dalton J, Finkelstein D, Lau CC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006; 24(12):1924–1931. PMID: 16567768.
Article3. Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011; 29(11):1408–1414. PMID: 20823417.
Article4. Kool M, Koster J, Bunt J, Hasselt NE, Lakeman A, van Sluis P, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008; 3(8):e3088. PMID: 18769486.
Article5. Cho YJ, Tsherniak A, Tamayo P, Santagata S, Ligon A, Greulich H, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011; 29(11):1424–1430. PMID: 21098324.
Article6. Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012; 123(4):465–472. PMID: 22134537.
Article7. Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016; 131(6):821–831. PMID: 27040285.
Article8. Gottardo NG, Hansford JR, McGlade JP, Alvaro F, Ashley DM, Bailey S, et al. Medulloblastoma Down Under 2013: a report from the third annual meeting of the International Medulloblastoma Working Group. Acta Neuropathol. 2014; 127(2):189–201. PMID: 24264598.
Article9. Northcott PA, Shih DJ, Remke M, Cho YJ, Kool M, Hawkins C, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012; 123(4):615–626. PMID: 22057785.
Article10. Lee JW, Lim DH, Sung KW, Cho HW, Ju HY, Hyun JK, et al. Promising survival rate but high incidence of treatment-related mortality after reduced-dose craniospinal radiotherapy and tandem high-dose chemotherapy in patients with high-risk medulloblastoma. Cancer Med. 2020; 9(16):5807–5818. PMID: 32608158.
Article11. von Bueren AO, Kortmann RD, von Hoff K, Friedrich C, Mynarek M, Müller K, et al. Treatment of children and adolescents with metastatic medulloblastoma and prognostic relevance of clinical and biologic parameters. J Clin Oncol. 2016; 34(34):4151–4160. PMID: 27863192.
Article12. Jakacki RI, Burger PC, Zhou T, Holmes EJ, Kocak M, Onar A, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children’s Oncology Group Phase I/II study. J Clin Oncol. 2012; 30(21):2648–2653. PMID: 22665539.
Article13. Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006; 7(10):813–820. PMID: 17012043.
Article14. Dufour C, Kieffer V, Varlet P, Raquin MA, Dhermain F, Puget S, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk medulloblastoma or supratentorial primitive neuro-ectodermic tumors. Pediatr Blood Cancer. 2014; 61(8):1398–1402. PMID: 24664937.
Article15. Bouffet E. Management of high-risk medulloblastoma. Neurochirurgie. 2021; 67(1):61–68. PMID: 31229532.
Article16. Sung KW, Lim DH, Son MH, Lee SH, Yoo KH, Koo HH, et al. Reduced-dose craniospinal radiotherapy followed by tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk medulloblastoma. Neuro-oncol. 2013; 15(3):352–359. PMID: 23258845.
Article17. Yoo HJ, Kim H, Park HJ, Kim DS, Ra YS, Shin HY. Neurocognitive function and health-related quality of life in pediatric Korean survivors of medulloblastoma. J Korean Med Sci. 2016; 31(11):1726–1734. PMID: 27709849.
Article18. Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B, Gururangan S, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016; 17(4):484–495. PMID: 26976201.
Article19. Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012; 30(26):3187–3193. PMID: 22851561.
Article20. Thompson EM, Bramall A, Herndon JE 2nd, Taylor MD, Ramaswamy V. The clinical importance of medulloblastoma extent of resection: a systematic review. J Neurooncol. 2018; 139(3):523–539. PMID: 29796724.
Article21. Cavalli FM, Remke M, Rampasek L, Peacock J, Shih DJ, Luu B, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell. 2017; 31(6):737–754.e6. PMID: 28609654.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medulloblastoma: Current Perspectives and Recent Advances
- Assessment of Prognosis and Risk Stratification in Coronary Artery Disease
- Risk Stratification of Acute Coronary Syndrome
- The Role of Carotid Ultrasound for Cardiovascular Risk Stratification beyond Traditional Risk Factors
- Risk stratification of thyroid nodules on ultrasonography with the French TI-RADS: description and reflections