J Korean Med Sci.
2022 Feb;37(5):e37. 10.3346/jkms.2022.37.e37.
Treatment of Human Immunodeficiency Virus-Associated Facial Lipoatrophy With Hyaluronic Acid Filler Mixed With Micronized Cross-Linked Acellular Dermal Matrix
- Affiliations
-
- 1Department of Dermatology, Severance Hospital, Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 2Scar Laser and Plastic Surgery Center, Yonsei Cancer Hospital, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Plastic and Reconstructive Surgery, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2525489
- DOI: http://doi.org/10.3346/jkms.2022.37.e37
Abstract
- Background
Human immunodeficiency virus (HIV)-associated facial lipoatrophy (FLA) is a stigmatizing side effect associated with the use of highly active antiretroviral therapy. We sought to evaluate the safety and efficacy of the hyaluronic acid filler mixed with micronized cross-linked acellular dermal matrix (HA/MADM) in HIV-associated FLA.
Methods
We conducted an open-label safety and efficacy study in patients with HIVassociated FLA. Fourteen patients received single injection of the HA/MADM, and 13 patients completed the 24-week follow-up evaluation. Treatment efficacy, safety, and patient and physician satisfaction were evaluated. Repeated measure analysis of variance with post-hoc analysis with the Wilcoxon signed rank test was performed to compare and incorporate parameters at each time point.
Results
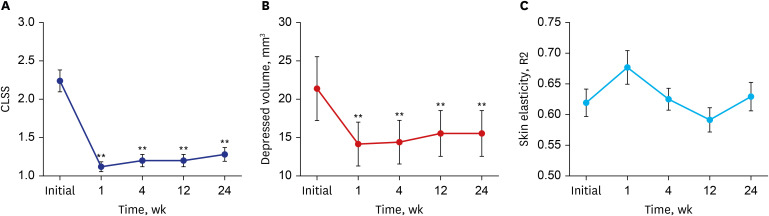
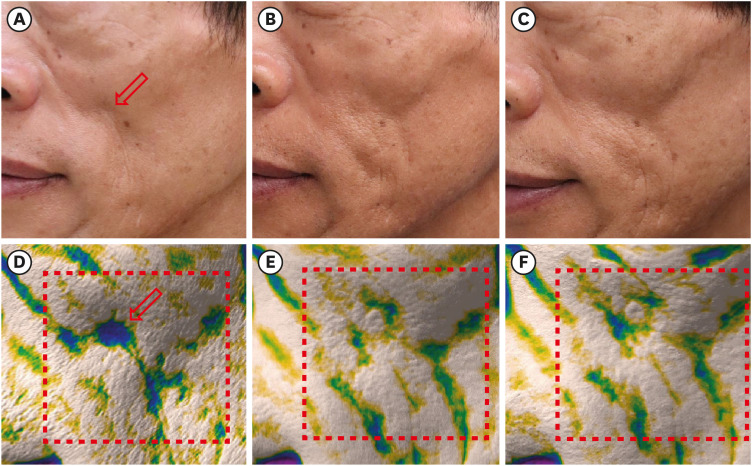
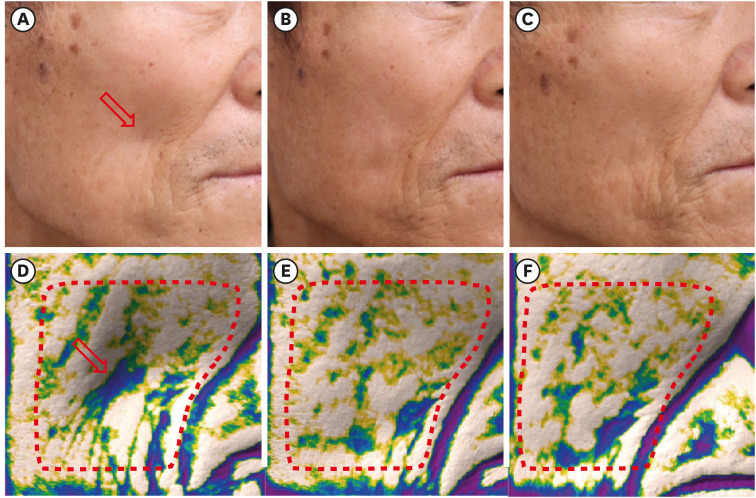
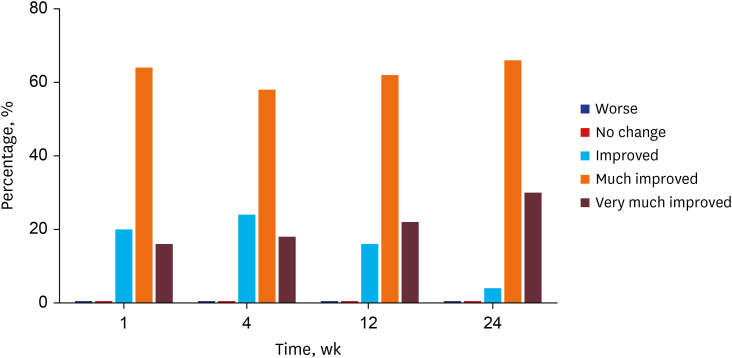
All 13 patients maintained a significant improvement of the Carruthers Lipoatrophy Severity Scale grade throughout the study period, along with improvement of the depressed volume due to lipoatrophy measured using a three-dimensional camera system. More than 80% of patients and physicians were satisfied with the treatment, and no treatment-related adverse events were reported, except for one case of transient subcutaneous nodule formation.
Conclusion
Our study findings suggest that injectable HA/MADM is a potentially effective and safe treatment option for treating HIV-positive patients with FLA.
Keyword
Figure
Reference
-
1. Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000; 14(3):F25–F32. PMID: 10716495.
Article2. Corless IB, Kirksey KM, Kemppainen J, Nicholas PK, McGibbon C, Davis SM, et al. Lipodystrophy-associated symptoms and medication adherence in HIV/AIDS. AIDS Patient Care STDS. 2005; 19(9):577–586. PMID: 16164384.
Article3. Rajagopalan R, Laitinen D, Dietz B. Impact of lipoatrophy on quality of life in HIV patients receiving anti-retroviral therapy. AIDS Care. 2008; 20(10):1197–1201. PMID: 18608076.
Article4. Jagdeo J, Ho D, Lo A, Carruthers A. A systematic review of filler agents for aesthetic treatment of HIV facial lipoatrophy (FLA). J Am Acad Dermatol. 2015; 73(6):1040–1054.e14. PMID: 26481056.
Article5. Koethe JR, Lagathu C, Lake JE, Domingo P, Calmy A, Falutz J, et al. HIV and antiretroviral therapy-related fat alterations. Nat Rev Dis Primers. 2020; 6(1):48. PMID: 32555389.
Article6. Ho D, Jagdeo J. Safety and efficacy of a volumizing hyaluronic acid filler for treatment of HIV-associated facial lipoatrophy. JAMA Dermatol. 2017; 153(1):61–65. PMID: 27806168.
Article7. Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013; 39(11):1602–1612. PMID: 24093664.
Article8. Shuck J, Iorio ML, Hung R, Davison SP. Autologous fat grafting and injectable dermal fillers for human immunodeficiency virus-associated facial lipodystrophy: a comparison of safety, efficacy, and long-term treatment outcomes. Plast Reconstr Surg. 2013; 131(3):499–506. PMID: 23142937.9. Moyle GJ, Brown S, Lysakova L, Barton SE. Long-term safety and efficacy of poly-L-lactic acid in the treatment of HIV-related facial lipoatrophy. HIV Med. 2006; 7(3):181–185. PMID: 16494632.
Article10. Carruthers A, Carruthers J. Evaluation of injectable calcium hydroxylapatite for the treatment of facial lipoatrophy associated with human immunodeficiency virus. Dermatol Surg. 2008; 34(11):1486–1499. PMID: 18811715.
Article11. Skeie L, Bugge H, Negaard A, Bergersen BM. Large particle hyaluronic acid for the treatment of facial lipoatrophy in HIV-positive patients: 3-year follow-up study. HIV Med. 2010; 11(3):170–177. PMID: 19780861.
Article12. Beasley KL, Weiss MA, Weiss RA. Hyaluronic acid fillers: a comprehensive review. Facial Plast Surg. 2009; 25(2):86–94. PMID: 19415575.
Article13. Lee JH, Kim HG, Lee WJ. Characterization and tissue incorporation of cross-linked human acellular dermal matrix. Biomaterials. 2015; 44:195–205. PMID: 25617138.
Article14. Park TH, Choi WY, Lee JH, Lee WJ. Micronized cross-linked human acellular dermal matrices: an effective scaffold for collagen synthesis and promising material for tissue augmentation. Tissue Eng Regen Med. 2017; 14(5):517–523. PMID: 30603506.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Linear Morphea (en Coup de Sabre) with Micronized Acellular Dermal Matrix Filler: A Case Report
- Facial Pseudocyst Caused by Hyaluronic Acid Filler Injection: A Case Report
- Basic rheology of dermal filler
- Effect of Avidin and Biotin in Attachment of Human Adipose Stem Cells to Micronized Acellular Dermal Matrix
- Hyaluronidase: An overview of its properties, applications, and side effects