Korean Circ J.
2022 Feb;52(2):110-122. 10.4070/kcj.2021.0191.
Stem Cell and Exosome Therapy in Pulmonary Hypertension
- Affiliations
-
- 1Gachon Cardiovascular Research Institute, Gachon University, Incheon, Korea
- 2Functional Cellular Networks Laboratory, Lee Gil Ya Cancer and Diabetes Institute, Gachon University, Incheon, Korea
- 3Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA
- 4Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA
- 5Division of Pediatric Cardiology, Department of Pediatrics, Gachon University Gil Medical Center, Incheon, Korea
- 6Department of Cardiovascular Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 7Department of Anatomy and Cell Biology, College of Medicine, Gachon University, Incheon, Korea
- 8Stanford University School of Medicine, Palo Alto, CA, USA
- KMID: 2525450
- DOI: http://doi.org/10.4070/kcj.2021.0191
Abstract
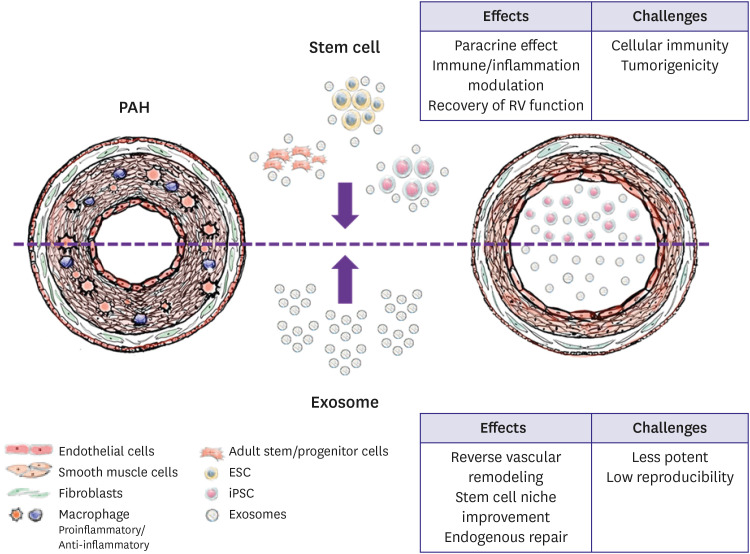
- Pulmonary hypertension is a rare and progressive illness with a devastating prognosis. Promising research efforts have advanced the understanding and recognition of the pathobiology of pulmonary hypertension. Despite remarkable achievements in terms of improving the survival rate, reducing disease progression, and enhancing quality of life, pulmonary arterial hypertension (PAH) is not completely curable. Therefore, an effective treatment strategy is still needed. Recently, many studies of the underlying molecular mechanisms and technological developments have led to new approaches and paradigms for PAH treatment. Management based on stem cells and related paracrine effects, epigenetic drugs and gene therapies has yielded prospective results for PAH treatment in preclinical research. Further trials are ongoing to optimize these important insights into clinical circumstances.
Figure
Cited by 2 articles
-
Epidemiology of PAH in Korea: An Analysis of the National Health Insurance Data, 2002–2018
Albert Youngwoo Jang, Hyeok-Hee Lee, Hokyou Lee, Hyeon Chang Kim, Wook-Jin Chung
Korean Circ J. 2023;53(5):313-327. doi: 10.4070/kcj.2022.0231.Current Trends and Movements in Managing Pulmonary Arterial Hypertension in Korea
Jaeho Seung, Hun-Jun Park
Korean Circ J. 2023;53(5):328-330. doi: 10.4070/kcj.2023.0039.
Reference
-
1. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019; 53:1801913. PMID: 30545968.
Article2. Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016; 4:306–322. PMID: 26975810.
Article3. Leber L, Beaudet A, Muller A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021; 11:2045894020977300. PMID: 33456755.
Article4. Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019; 53:1801887. PMID: 30545970.
Article5. Guignabert C, Tu L, Girerd B, et al. New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication. Chest. 2015; 147:529–537. PMID: 25644906.
Article6. Frid MG, Thurman JM, Hansen KC, Maron BA, Stenmark KR. Inflammation, immunity, and vascular remodeling in pulmonary hypertension; evidence for complement involvement? Glob Cardiol Sci Pract. 2020; 2020:e202001. PMID: 32478115.
Article7. Yeo Y, Yi ES, Kim JM, et al. FGF12 (fibroblast growth factor 12) inhibits vascular smooth muscle cell remodeling in pulmonary arterial hypertension. Hypertension. 2020; 76:1778–1786. PMID: 33100045.
Article8. Ahn KJ, Jang AY, Park SJ, Chung WJ. 15 years journey of idiopathic pulmonary arterial hypertension with BMPR2 mutation. Clin Hypertens. 2019; 25:22. PMID: 31583114.
Article9. Jang AY, Kim BG, Kwon S, et al. Prevalence and clinical features of bone morphogenetic protein receptor type 2 mutation in Korean idiopathic pulmonary arterial hypertension patients: the PILGRIM explorative cohort. PLoS One. 2020; 15:e0238698. PMID: 32966279.
Article10. Lee H, Yeom A, Kim KC, Hong YM. Effect of ambrisentan therapy on the expression of endothelin receptor, endothelial nitric oxide synthase and NADPH oxidase 4 in monocrotaline-induced pulmonary arterial hypertension rat model. Korean Circ J. 2019; 49:866–876. PMID: 31165592.
Article11. Jang AY, Kim S, Park SJ, et al. A nationwide multicenter registry and biobank program for deep phenotyping of idiopathic and hereditary pulmonary arterial hypertension in Korea: the PAH platform for deep phenotyping in Korean subjects (PHOENIKS) cohort. Clin Hypertens. 2019; 25:21. PMID: 31534782.
Article12. Sitbon O, Gomberg-Maitland M, Granton J, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019; 53:1801908. PMID: 30545975.
Article13. Berghausen EM, Feik L, Zierden M, Vantler M, Rosenkranz S. Key inflammatory pathways underlying vascular remodeling in pulmonary hypertension. Herz. 2019; 44:130–137. PMID: 30847510.
Article14. Frid MG, Brunetti JA, Burke DL, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006; 168:659–669. PMID: 16436679.
Article15. Yu YA, Malakhau Y, Yu CA, et al. Nonclassical monocytes sense hypoxia, regulate pulmonary vascular remodeling, and promote pulmonary hypertension. J Immunol. 2020; 204:1474–1485. PMID: 31996456.
Article16. Fazal S, Bisserier M, Hadri L. Molecular and genetic profiling for precision medicines in pulmonary arterial hypertension. Cells. 2021; 10:638. PMID: 33805595.
Article17. Pu X, Du L, Hu Y, Fan Y, Xu Q. Stem/progenitor cells and pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol. 2021; 41:167–178. PMID: 33028095.
Article18. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019; 4:22. PMID: 31815001.
Article19. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014; 15:1009–1016. PMID: 25329189.
Article20. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147. PMID: 10102814.
Article21. Mohsin S, Houser SR. Cortical bone derived stem cells for cardiac wound healing. Korean Circ J. 2019; 49:314–325. PMID: 30808081.
Article22. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8:315–317. PMID: 16923606.
Article23. Bui TV, Hwang JW, Lee JH, Park HJ, Ban K. Challenges and limitations of strategies to promote therapeutic potential of human mesenchymal stem cells for cell-based cardiac repair. Korean Circ J. 2021; 51:97–113. PMID: 33525065.
Article24. Roobrouck VD, Ulloa-Montoya F, Verfaillie CM. Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res. 2008; 314:1937–1944. PMID: 18439579.
Article25. Jun D, Garat C, West J, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells. 2011; 29:725–735. PMID: 21312316.
Article26. Bordenave J, Tu L, Berrebeh N, et al. Lineage tracing reveals the dynamic contribution of pericytes to the blood vessel remodeling in pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2020; 40:766–782. PMID: 31969018.
Article27. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676. PMID: 16904174.
Article28. Morita Y, Okura H, Matsuyama A. Patent application trends of induced pluripotent stem cell technologies in the United States, Japanese, and European applications. Biores Open Access. 2019; 8:45–58. PMID: 30906670.
Article29. Mandai M, Kurimoto Y, Takahashi M. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017; 377:792–793.
Article30. Glassberg MK, Minkiewicz J, Toonkel RL, et al. Allogeneic human mesenchymal stem cells in patients with idiopathic pulmonary fibrosis via intravenous delivery (AETHER): a phase I safety clinical trial. Chest. 2017; 151:971–981. PMID: 27890713.
Article31. Braza F, Dirou S, Forest V, et al. Mesenchymal stem cells induce suppressive macrophages through phagocytosis in a mouse model of asthma. Stem Cells. 2016; 34:1836–1845. PMID: 26891455.
Article32. Oh S, Jang AY, Chae S, et al. Comparative analysis on the anti-inflammatory/immune effect of mesenchymal stem cell therapy for the treatment of pulmonary arterial hypertension. Sci Rep. 2021; 11:2012. PMID: 33479312.
Article33. Huang WC, Ke MW, Cheng CC, et al. Therapeutic benefits of induced pluripotent stem cells in monocrotaline-induced pulmonary arterial hypertension. PLoS One. 2016; 11:e0142476. PMID: 26840075.
Article34. Gu M, Shao NY, Sa S, et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell. 2017; 20:490–504.e5. PMID: 28017794.
Article35. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020; 367:eaau6977. PMID: 32029601.
Article36. van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006; 140:13–21. PMID: 16877764.
Article37. Glembotski CC. Expanding the paracrine hypothesis of stem cell-mediated repair in the heart: when the unconventional becomes conventional. Circ Res. 2017; 120:772–774. PMID: 28254800.
Article38. Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res (Amst). 2007; 1:129–137.
Article39. Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res (Amst). 2010; 4:214–222.
Article40. Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011; 9:12. PMID: 21569606.
Article41. Wang K, Jiang Z, Webster KA, et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl Med. 2017; 6:209–222. PMID: 28170197.
Article42. Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012; 126:2601–2611. PMID: 23114789.
Article43. Zhang S, Liu X, Ge LL, et al. Mesenchymal stromal cell-derived exosomes improve pulmonary hypertension through inhibition of pulmonary vascular remodeling. Respir Res. 2020; 21:71. PMID: 32192495.
Article44. Sun F, Wang G, Pradhan A, et al. Nanoparticle delivery of STAT3 alleviates pulmonary hypertension in a mouse model of alveolar capillary dysplasia. Circulation. 2021; 144:539–555. PMID: 34111939.
Article45. Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015; 117:52–64. PMID: 25904597.
Article46. Wang Y, Zhang L, Li Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015; 192:61–69. PMID: 26000464.
Article47. Jung JH, Fu X, Yang PC. Exosomes generated from iPSC-derivatives: new direction for stem cell therapy in human heart diseases. Circ Res. 2017; 120:407–417. PMID: 28104773.48. Ikeda G, Santoso MR, Tada Y, et al. Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J Am Coll Cardiol. 2021; 77:1073–1088. PMID: 33632482.
Article49. Jung JH, Ikeda G, Tada Y, et al. miR-106a-363 cluster in extracellular vesicles promotes endogenous myocardial repair via Notch3 pathway in ischemic heart injury. Basic Res Cardiol. 2021; 116:19. PMID: 33742276.
Article50. Lee JR, Park BW, Kim J, et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv. 2020; 6:eaaz0952. PMID: 32494669.
Article51. de Mendonça L, Felix NS, Blanco NG, et al. Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension. Stem Cell Res Ther. 2017; 8:220. PMID: 28974252.
Article52. Liang OD, Mitsialis SA, Chang MS, et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011; 29:99–107. PMID: 20957739.
Article53. Jiang L, Song XH, Liu P, et al. Platelet-mediated mesenchymal stem cells homing to the lung reduces monocrotaline-induced rat pulmonary hypertension. Cell Transplant. 2012; 21:1463–1475. PMID: 22525351.
Article54. Kanki-Horimoto S, Horimoto H, Mieno S, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006; 114:I181–I185. PMID: 16820570.
Article55. Luan Y, Zhang X, Qi TG, Cheng GH, Sun C, Kong F. Long-term research of stem cells in monocrotaline-induced pulmonary arterial hypertension. Clin Exp Med. 2014; 14:439–446. PMID: 23996433.
Article56. Takemiya K, Kai H, Yasukawa H, Tahara N, Kato S, Imaizumi T. Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic Res Cardiol. 2010; 105:409–417. PMID: 19838762.
Article57. Umar S, de Visser YP, Steendijk P, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009; 297:H1606–H1616. PMID: 19783775.
Article58. Liu J, Han Z, Han Z, He Z. Mesenchymal stem cells suppress CaN/NFAT expression in the pulmonary arteries of rats with pulmonary hypertension. Exp Ther Med. 2015; 10:1657–1664. PMID: 26640533.
Article59. Alencar AK, Pimentel-Coelho PM, Montes GC, et al. Human mesenchymal stem cell therapy reverses Su5416/hypoxia-induced pulmonary arterial hypertension in mice. Front Pharmacol. 2018; 9:1395. PMID: 30574088.
Article60. Lee H, Kim KC, Choi SJ, Hong YM. Optimal dose and timing of umbilical stem cells treatment in pulmonary arterial hypertensive rats. Yonsei Med J. 2017; 58:570–580. PMID: 28332363.
Article61. Zhang Y, Liao S, Yang M, et al. Improved cell survival and paracrine capacity of human embryonic stem cell-derived mesenchymal stem cells promote therapeutic potential for pulmonary arterial hypertension. Cell Transplant. 2012; 21:2225–2239. PMID: 22776744.
Article62. Lopatina T, Bruno S, Tetta C, Kalinina N, Porta M, Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014; 12:26. PMID: 24725987.
Article63. Zhang C, Wang P, Mohammed A, et al. Function of adipose-derived mesenchymal stem cells in monocrotaline-induced pulmonary arterial hypertension through miR-191 via regulation of BMPR2. BioMed Res Int. 2019; 2019:2858750. PMID: 31119161.
Article64. Klinger JR, Pereira M, Del Tatto M, et al. Mesenchymal stem cell extracellular vesicles reverse sugen/hypoxia pulmonary hypertension in rats. Am J Respir Cell Mol Biol. 2020; 62:577–587. PMID: 31721618.
Article65. Aliotta JM, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016; 110:319–330. PMID: 26980205.
Article66. Komaki M, Numata Y, Morioka C, et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017; 8:219. PMID: 28974256.
Article67. Salomon C, Ryan J, Sobrevia L, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013; 8:e68451. PMID: 23861904.
Article