Diabetes Metab J.
2022 Jan;46(1):117-128. 10.4093/dmj.2020.0275.
Influence of Glucose Fluctuation on Peripheral Nerve Damage in Streptozotocin-Induced Diabetic Rats
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Research Institute of Clinical Medicine of Jeonbuk National University Medical School-Biomedical Research Institute of Jeonbuk National University Hospital, Jeonju, Korea
- KMID: 2525131
- DOI: http://doi.org/10.4093/dmj.2020.0275
Abstract
- Background
It is unclear whether glycemic variability (GV) is a risk factor for diabetic peripheral neuropathy (DPN), and whether control of GV is beneficial for DPN. The purpose of this study was to investigate the effect of GV on peripheral nerve damage by inducing glucose fluctuation in streptozotocin-induced diabetic rats.
Methods
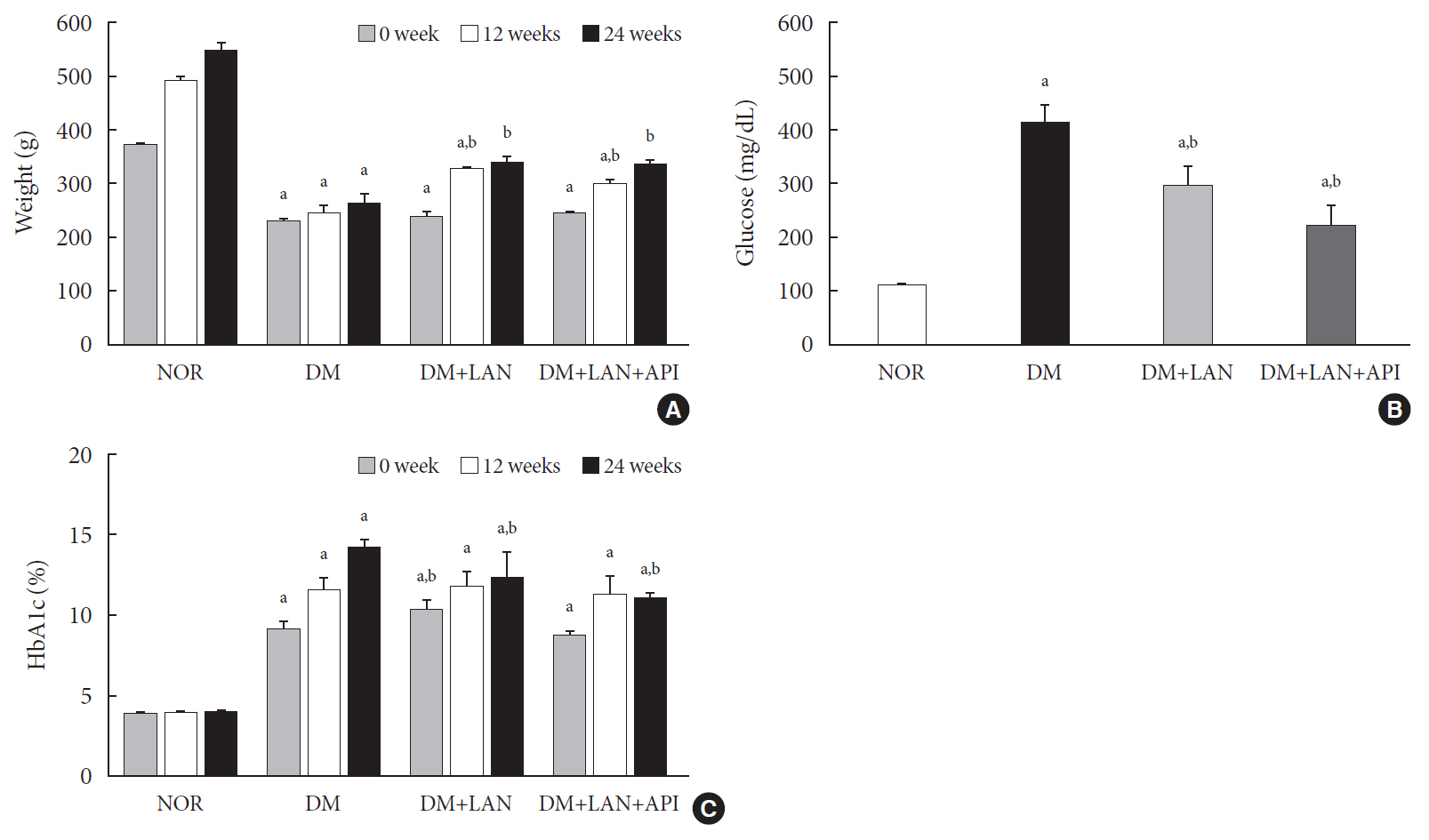
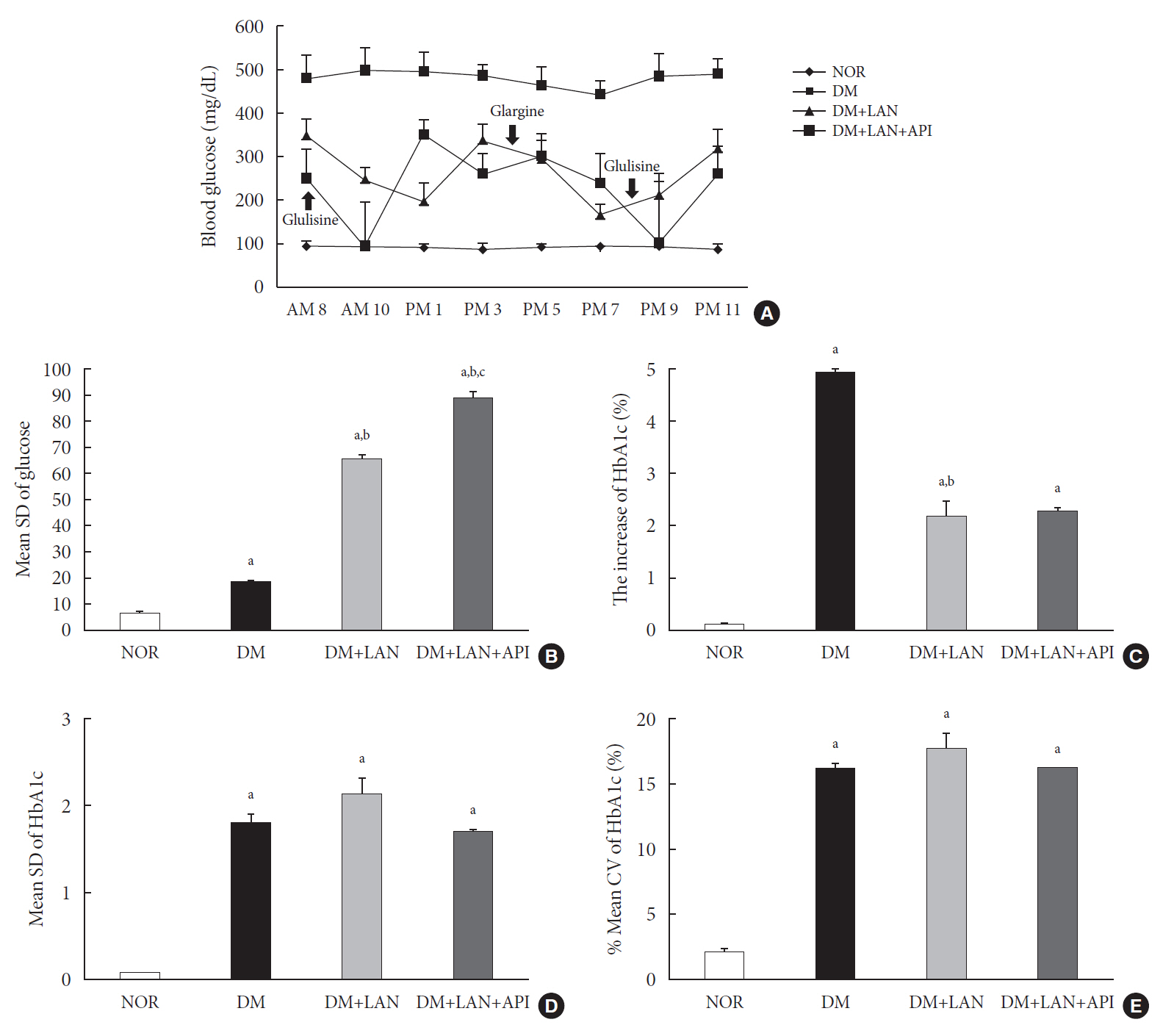
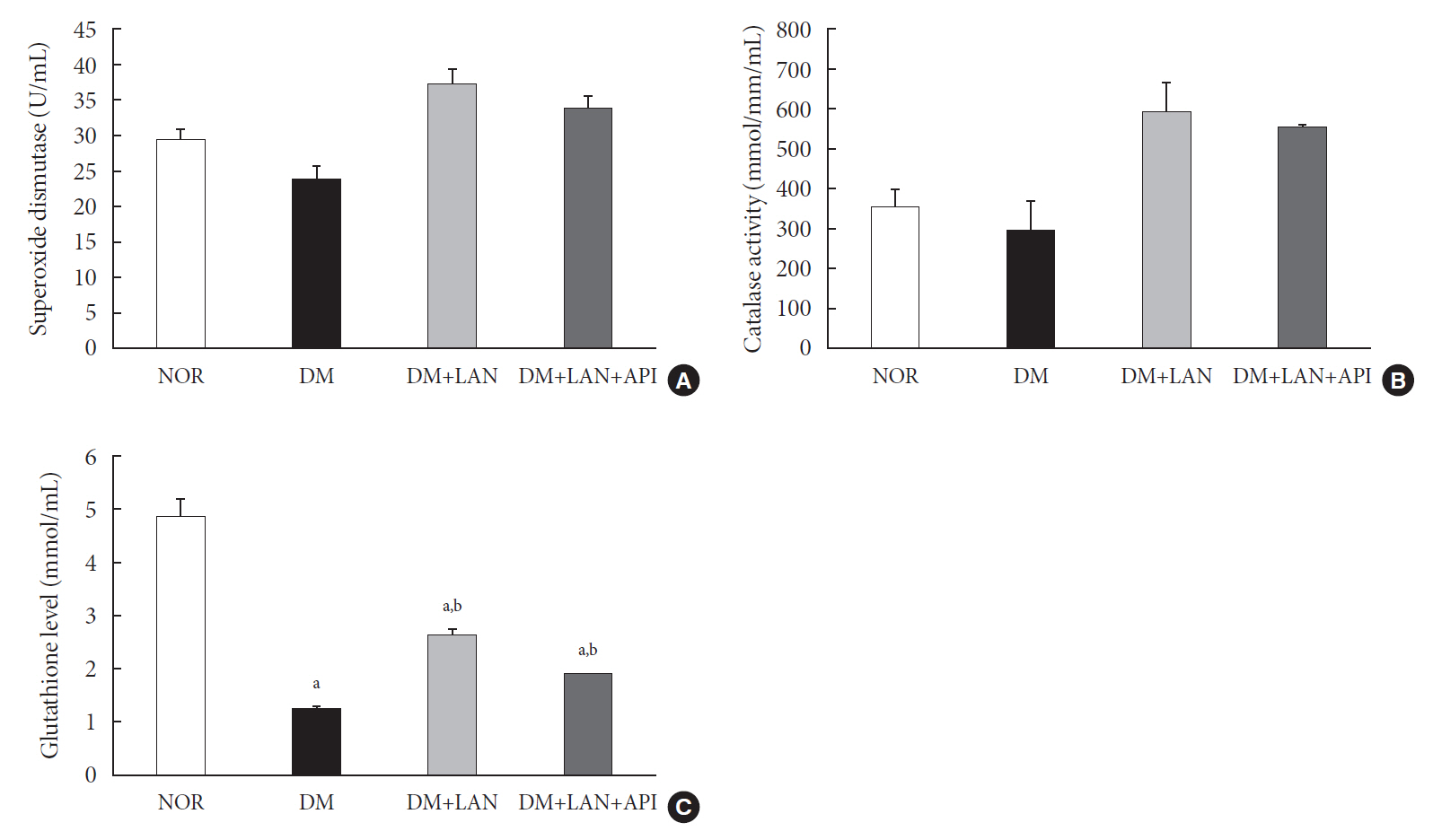
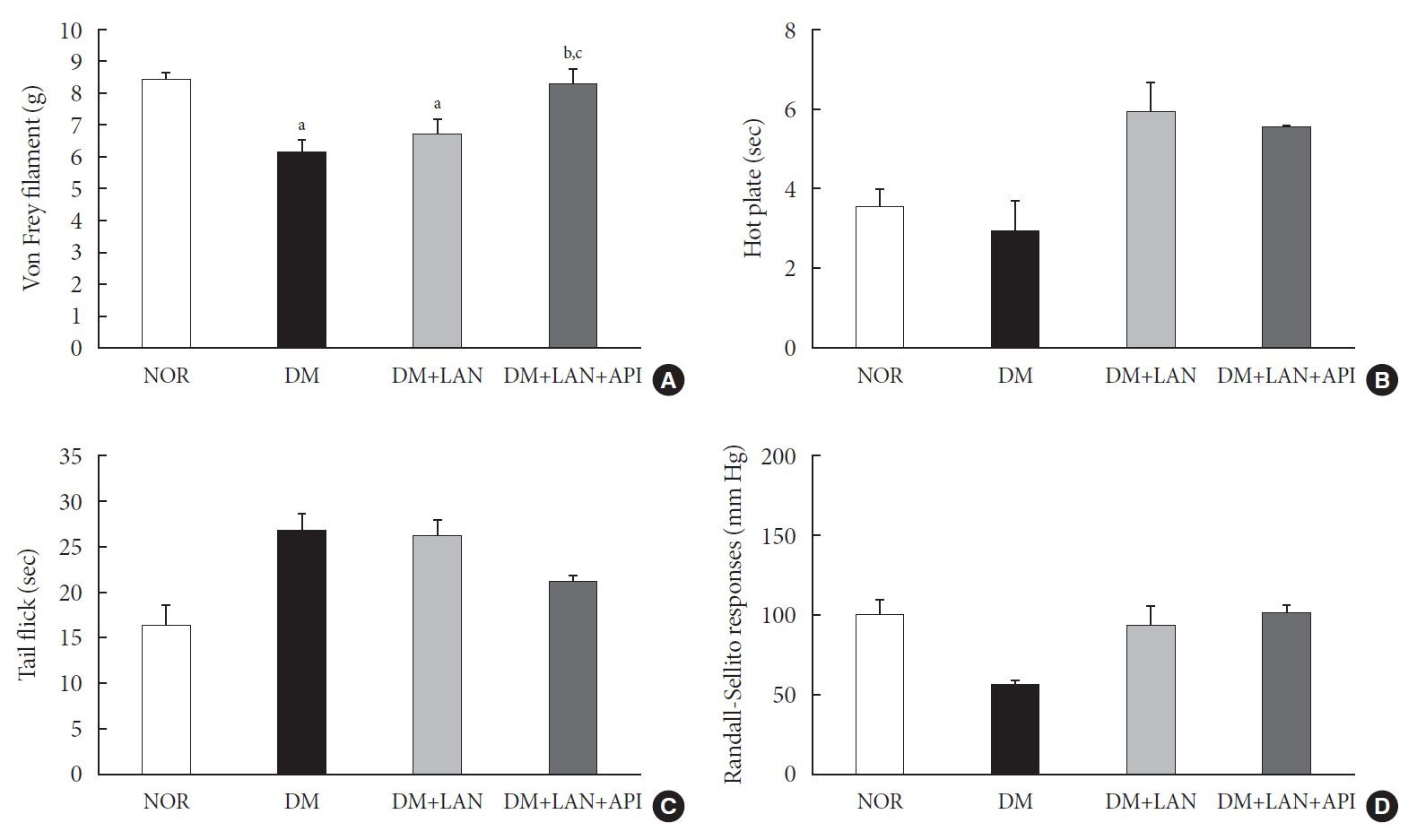
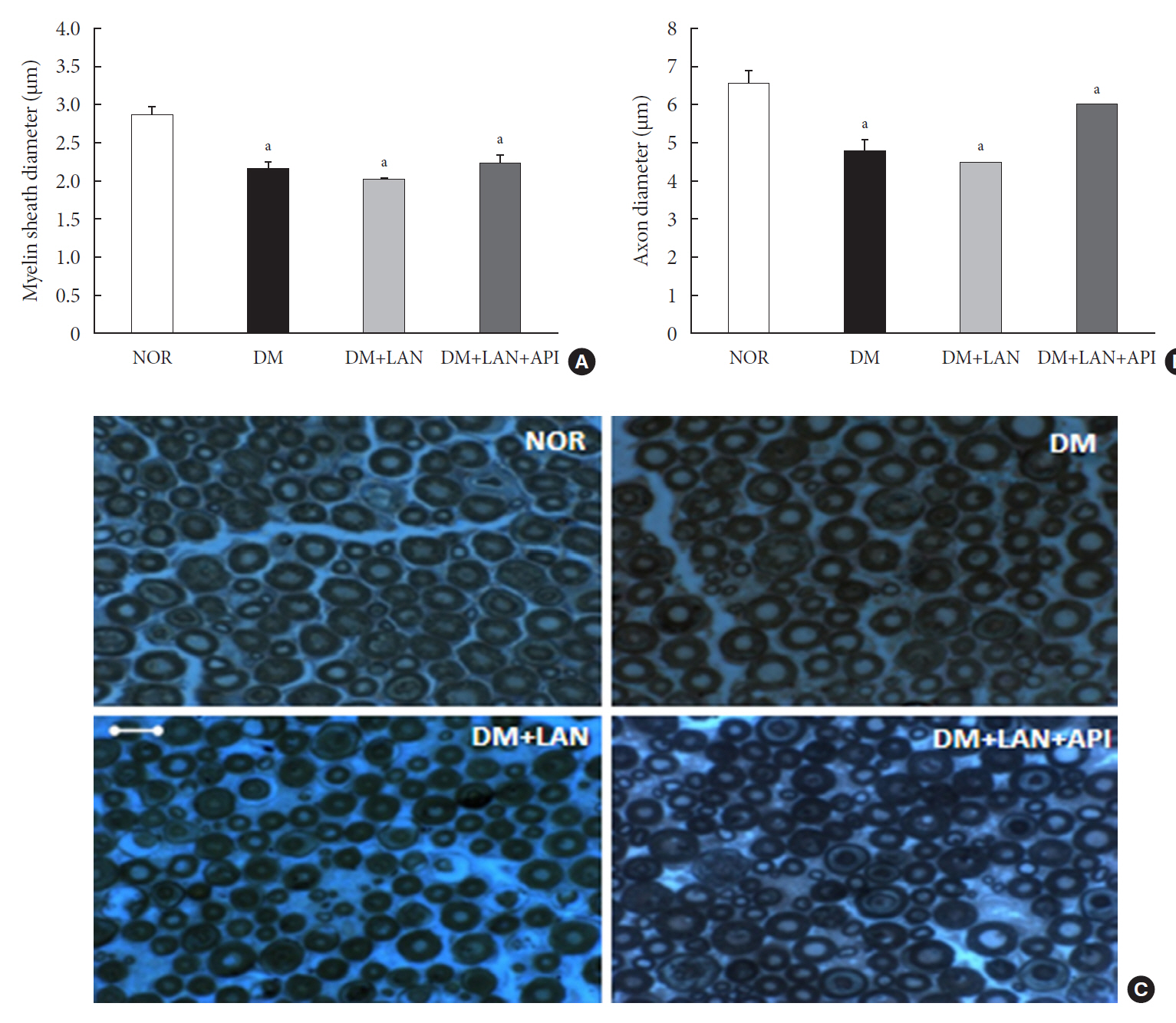
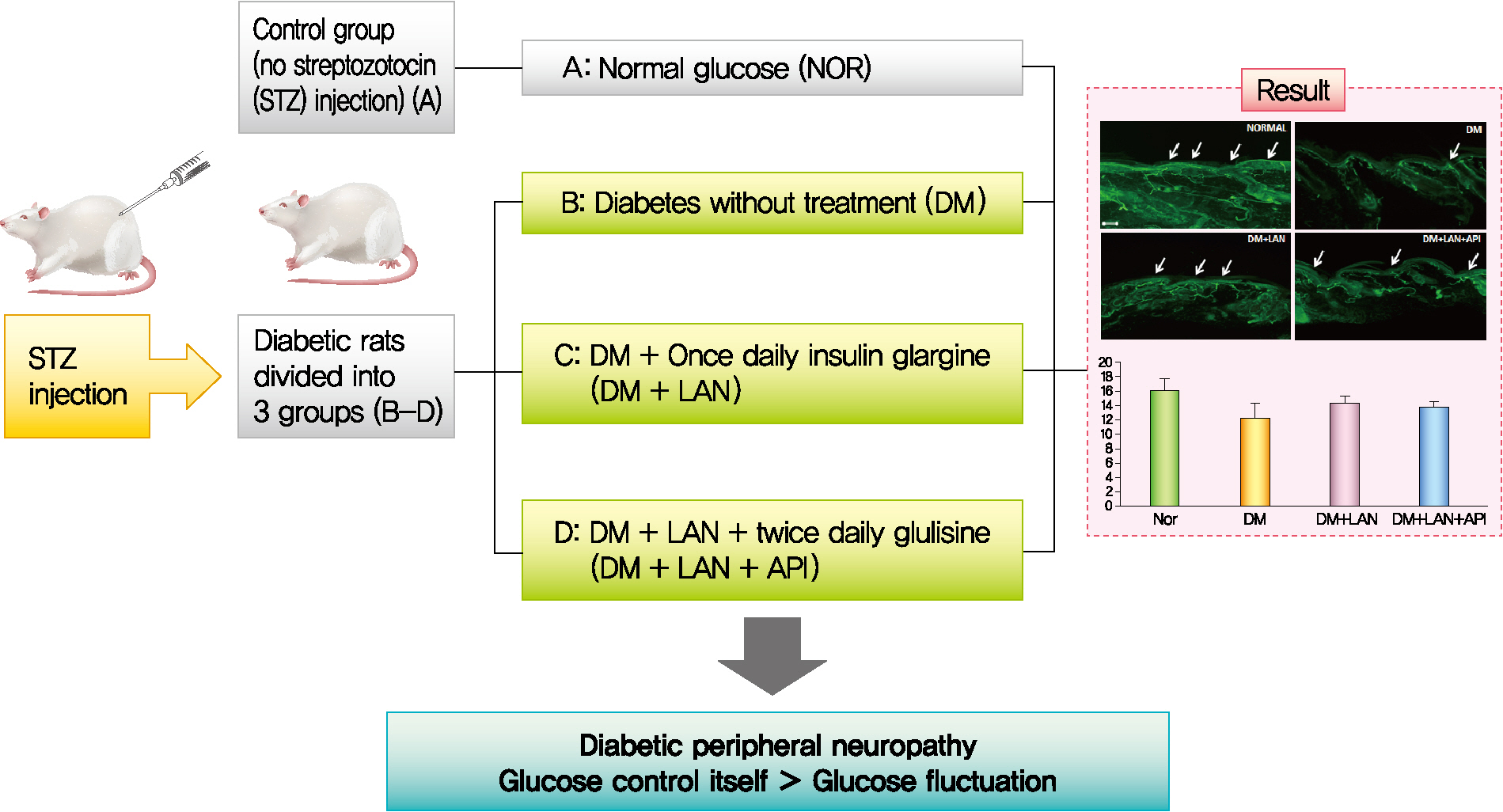
Rats were divided into four groups: normal (normal glucose group [NOR]), diabetes without treatment (sustained severe hyperglycemia group; diabetes mellitus [DM]), diabetes+once daily insulin glargine (stable hyperglycemia group; DM+LAN), and diabetes+once daily insulin glargine with twice daily insulin glulisine (unstable glucose fluctuation group; DM+Lantus [LAN]+Apidra [API]). We measured anti-oxidant enzyme levels and behavioral responses against tactile, thermal, and pressure stimuli in the plasma of rats. We also performed a quantitative comparison of cutaneous and sciatic nerves according to glucose fluctuation.
Results
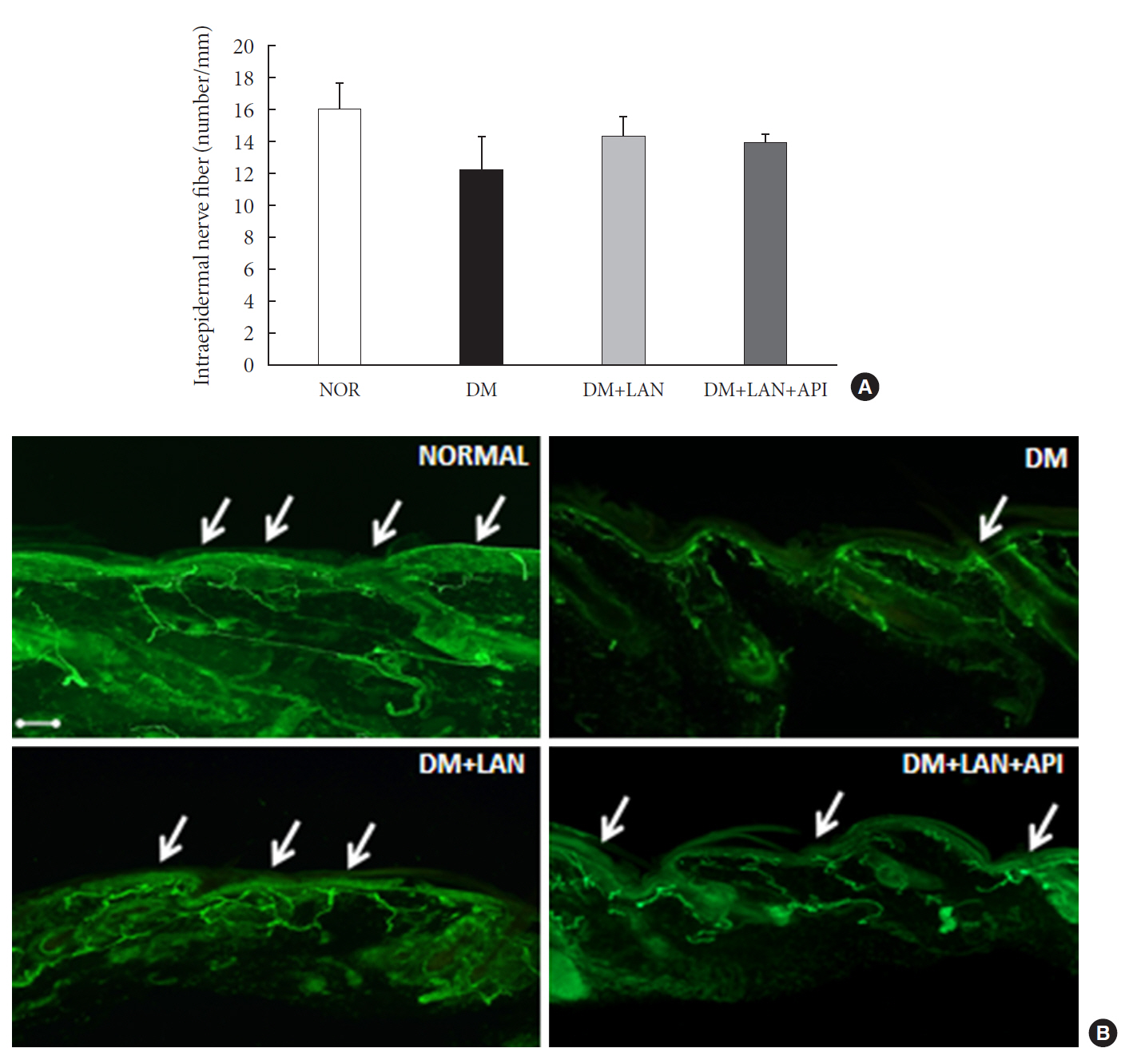
At week 24, intraepidermal nerve fiber density was less reduced in the insulin-administered groups compared to the DM group (P<0.05); however, a significant difference was not observed between the DM+LAN and DM+LAN+API groups irrespective of glucose fluctuation (P>0.05; 16.2±1.6, 12.4±2.0, 14.3±0.9, and 13.9±0.6 for NOR, DM, DM+LAN, and DM+LAN+API, respectively). The DM group exhibited significantly decreased glutathione levels compared to the insulin-administered groups (2.64±0.10 μmol/mL, DM+LAN; 1.93±0.0 μmol/mL, DM+LAN+API vs. 1.25±0.04 μmol/mL, DM; P<0.05).
Conclusion
Our study suggests that glucose control itself is more important than glucose fluctuation in the prevention of peripheral nerve damage, and intra-day glucose fluctuation has a limited effect on the progression of peripheral neuropathy in rats with diabetes.
Figure
Reference
-
1. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993; 36:150–4.
Article2. Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 (3rd and last part). Diabete Metab. 1977; 3:245–56.3. Yagihashi S, Mizukami H, Sugimoto K. Mechanism of diabetic neuropathy: where are we now and where to go? J Diabetes Investig. 2011; 2:18–32.
Article4. Sabanayagam C, Liew G, Tai ES, Shankar A, Lim SC, Subramaniam T, et al. Relationship between glycated haemoglobin and microvascular complications: is there a natural cut-off point for the diagnosis of diabetes? Diabetologia. 2009; 52:1279–89.
Article5. Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015; 39:273–82.
Article6. Satya Krishna SV, Kota SK, Modi KD. Glycemic variability: clinical implications. Indian J Endocrinol Metab. 2013; 17:611–9.
Article7. Sartore G, Chilelli NC, Burlina S, Di Stefano P, Piarulli F, Fedele D, et al. The importance of HbA1c and glucose variability in patients with type 1 and type 2 diabetes: outcome of continuous glucose monitoring (CGM). Acta Diabetol. 2012; 49 Suppl 1:S153–60.
Article8. Kalopita S, Liatis S, Thomakos P, Vlahodimitris I, Stathi C, Katsilambros N, et al. Relationship between autonomic nervous system function and continuous interstitial glucose measurement in patients with type 2 diabetes. J Diabetes Res. 2014; 2014:835392.
Article9. Jun JE, Jin SM, Baek J, Oh S, Hur KY, Lee MS, et al. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015; 14:70.
Article10. Yalcin I, Charlet A, Freund-Mercier MJ, Barrot M, Poisbeau P. Differentiating thermal allodynia and hyperalgesia using dynamic hot and cold plate in rodents. J Pain. 2009; 10:767–73.
Article11. Jin HY, Kang SM, Liu WJ, Song CH, Lee KA, Baek HS, et al. Comparison of peripheral nerve damages according to glucose control timing in experimental diabetes. Exp Clin Endocrinol Diabetes. 2012; 120:451–9.
Article12. Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, et al. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010; 17:903–12.13. Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005; 12:747–58.
Article14. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003; 52:2795–804.15. Kim MJ, Jung HS, Hwang-Bo Y, Cho SW, Jang HC, Kim SY, et al. Evaluation of 1,5-anhydroglucitol as a marker for glycemic variability in patients with type 2 diabetes mellitus. Acta Diabetol. 2013; 50:505–10.
Article16. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295:1681–7.
Article17. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008; 57:1349–54.
Article18. Bragd J, Adamson U, Backlund LB, Lins PE, Moberg E, Oskarsson P. Can glycaemic variability, as calculated from blood glucose self-monitoring, predict the development of complications in type 1 diabetes over a decade? Diabetes Metab. 2008; 34(6 Pt 1):612–6.19. Gimeno-Orna JA, Castro-Alonso FJ, Boned-Juliani B, Lou-Arnal LM. Fasting plasma glucose variability as a risk factor of retinopathy in type 2 diabetic patients. J Diabetes Complications. 2003; 17:78–81.
Article20. Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, et al. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013; 36:1026–32.
Article21. Wang X, Zhao X, Dorje T, Yan H, Qian J, Ge J. Glycemic variability predicts cardiovascular complications in acute myocardial infarction patients with type 2 diabetes mellitus. Int J Cardiol. 2014; 172:498–500.
Article22. Salardi S, Zucchini S, Santoni R, Ragni L, Gualandi S, Cicognani A, et al. The glucose area under the profiles obtained with continuous glucose monitoring system relationships with HbA(lc) in pediatric type 1 diabetic patients. Diabetes Care. 2002; 25:1840–4.
Article23. Oyibo SO, Prasad YD, Jackson NJ, Jude EB, Boulton AJ. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: a pilot study. Diabet Med. 2002; 19:870–3.
Article24. Frontoni S, Di Bartolo P, Avogaro A, Bosi E, Paolisso G, Ceriello A. Glucose variability: an emerging target for the treatment of diabetes mellitus. Diabetes Res Clin Pract. 2013; 102:86–95.
Article25. Lin CC, Chen CC, Chen FN, Li CI, Liu CS, Lin WY, et al. Risks of diabetic nephropathy with variation in hemoglobin A1c and fasting plasma glucose. Am J Med. 2013; 126:1017.26. Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008; 31:2198–202.27. Jin HY, Lee KA, Park TS. The impact of glycemic variability on diabetic peripheral neuropathy. Endocrine. 2016; 53:643–8.
Article28. Xu F, Zhao LH, Su JB, Chen T, Wang XQ, Chen JF, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014; 6:139.
Article29. Fleischer J. Diabetic autonomic imbalance and glycemic variability. J Diabetes Sci Technol. 2012; 6:1207–15.
Article30. Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 2008; 110:351–62.
Article31. Katulanda P, Ranasinghe P, Jayawardena R, Constantine GR, Sheriff MH, Matthews DR. The prevalence, patterns and predictors of diabetic peripheral neuropathy in a developing country. Diabetol Metab Syndr. 2012; 4:21.
Article32. The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia. 1998; 41:416–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Empagliflozin, a Selective Sodium-Glucose Cotransporter 2 Inhibitor, on Kidney and Peripheral Nerves in Streptozotocin-Induced Diabetic Rats
- Effects of Polygonatum odoratum on In vivo Insulin Activity in Streptozotocin-Induced Diabetic Rats
- Response of peripheral nerve to transient ischemia in streptozotocin-induced diabetic rats
- The Role of the Central Parasympathetic Nervous System in Modulating Glucose Metabolism in Streptozotocin-induced Diabetic Rats
- Pattern of Stress-Induced Hyperglycemia according to Type of Diabetes: A Predator Stress Model