Diabetes Metab J.
2022 Jan;46(1):63-70. 10.4093/dmj.2020.0253.
Increasing Age Associated with Higher Dipeptidyl Peptidase-4 Inhibition Rate Is a Predictive Factor for Efficacy of Dipeptidyl Peptidase-4 Inhibitors
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Life Sciences, LG Chem Ltd., Seoul, Korea
- 4Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2525126
- DOI: http://doi.org/10.4093/dmj.2020.0253
Abstract
- Background
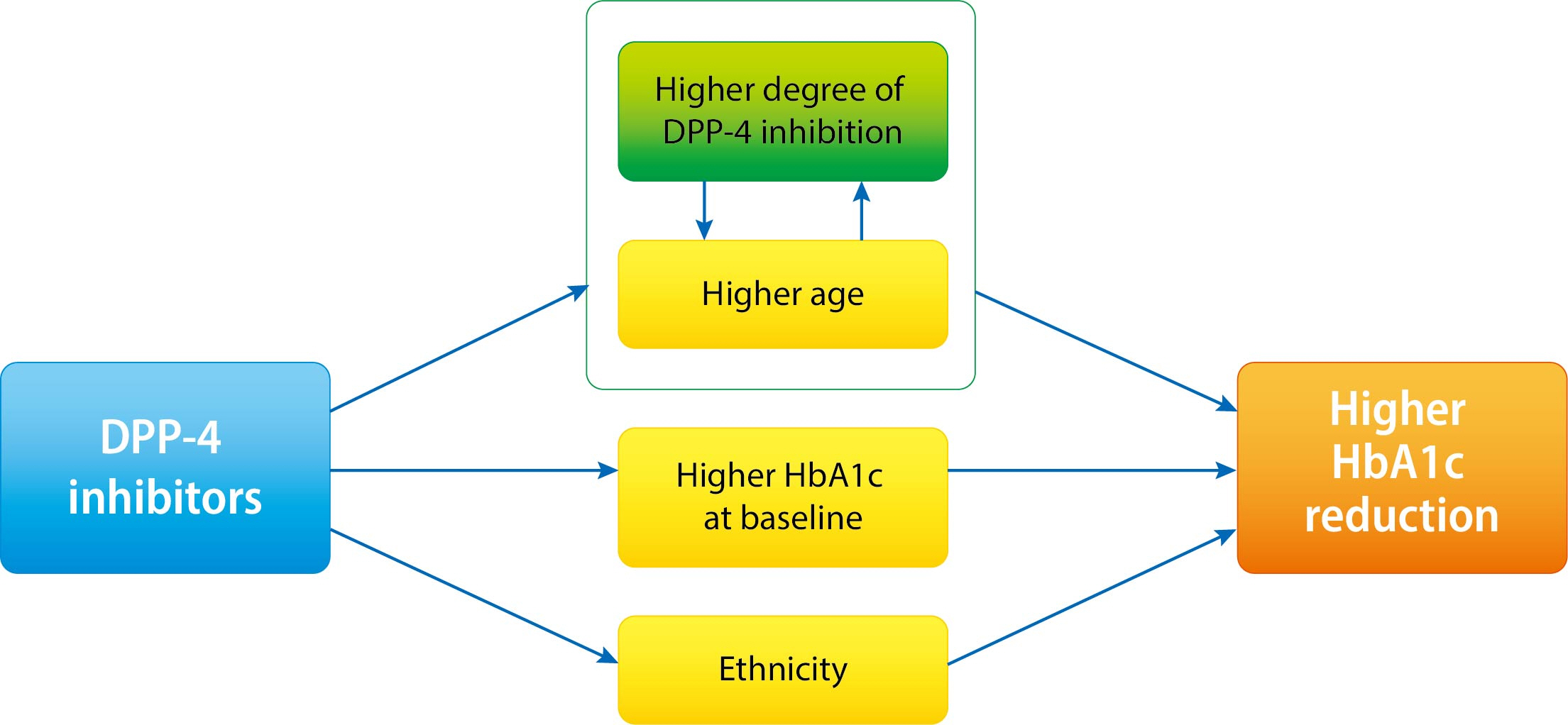
It is not known which type 2 diabetes mellitus (T2DM) patients would most benefit from dipeptidyl peptidase-4 (DPP-4) inhibitor treatment. We aimed to investigate the predictors of response to DPP-4 inhibitors considering degree of DPP-4 inhibition.
Methods
This study is a post hoc analysis of a 24-week, randomized, double-blind, phase III trial that compared the efficacy and safety of a DPP-4 inhibitor (gemigliptin vs. sitagliptin) in patients with T2DM. Subjects were classified into tertiles of T1 <65.26%, T2=65.26%–76.35%, and T3 ≥76.35% by DPP-4 inhibition. We analyzed the change from baseline in glycosylated hemoglobin (HbA1c) according to DPP-4 inhibition with multiple linear regression adjusting for age, ethnicity, body mass index, baseline HbA1c, and DPP-4 activity at baseline.
Results
The mean age was greater in the high tertile group compared with the low tertile group (T1: 49.8±8.3 vs. T2: 53.1±10.5 vs. T3: 55.3±9.5, P<0.001) of DPP-4 inhibition. Although HbA1c at baseline was not different among tertiles of DPP-4 inhibition (P=0.398), HbA1c after 24-week treatment was lower in the higher tertile compares to the lower tertile (T1: 7.30%±0.88% vs. T2: 7.12%±0.78% vs. T3: 7.00%±0.78%, P=0.021). In multiple regression analysis, DPP-4 enzyme inhibition rate was not a significant determent for HbA1c reduction due to age. In subgroup analysis by tertile of DPP-4 inhibition, age was the only significant predictor and only in the highest tertile (R2=0.281, B=–0.014, P=0.024).
Conclusion
This study showed that HbA1c reduction by DPP-4 inhibitor was associated with increasing age, and this association was linked with higher DPP-4 inhibition.
Figure
Reference
-
1. Ko SH, Han K, Lee YH, Noh J, Park CY, Kim DJ, et al. Past and current status of adult type 2 diabetes mellitus management in Korea: a National Health Insurance Service database analysis. Diabetes Metab J. 2018; 42:93–100.
Article2. Roder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016; 48:e219.
Article3. Monami M, Cremasco F, Lamanna C, Marchionni N, Mannucci E. Predictors of response to dipeptidyl peptidase-4 inhibitors: evidence from randomized clinical trials. Diabetes Metab Res Rev. 2011; 27:362–72.
Article4. Bihan H, Ng WL, Magliano DJ, Shaw JE. Predictors of efficacy of GLP-1 agonists and DPP-4 inhibitors: a systematic review. Diabetes Res Clin Pract. 2016; 121:27–34.
Article5. Dennis JM, Shields BM, Hill AV, Knight BA, McDonald TJ, Rodgers LR, et al. Precision medicine in type 2 diabetes: clinical markers of insulin resistance are associated with altered short- and long-term glycemic response to DPP-4 inhibitor therapy. Diabetes Care. 2018; 41:705–12.
Article6. Esposito K, Chiodini P, Maiorino MI, Capuano A, Cozzolino D, Petrizzo M, et al. A nomogram to estimate the HbA1c response to different DPP-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of 98 trials with 24 163 patients. BMJ Open. 2015; 5:e005892.
Article7. Deacon CF. Physiology and pharmacology of DPP-4 in glucose homeostasis and the treatment of type 2 diabetes. Front Endocrinol (Lausanne). 2019; 10:80.
Article8. Tatosian DA, Guo Y, Schaeffer AK, Gaibu N, Popa S, Stoch A, et al. Dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes treated with saxagliptin, sitagliptin, or vildagliptin. Diabetes Ther. 2013; 4:431–42.
Article9. Gibbs JP, Fredrickson J, Barbee T, Correa I, Smith B, Lin SL, et al. Quantitative model of the relationship between dipeptidyl peptidase-4 (DPP-4) inhibition and response: meta-analysis of alogliptin, saxagliptin, sitagliptin, and vildagliptin efficacy results. J Clin Pharmacol. 2012; 52:1494–505.
Article10. Yang SJ, Min KW, Gupta SK, Park JY, Shivane VK, Pitale SU, et al. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes Metab. 2013; 15:410–6.
Article11. Kim SH, Yoo JH, Lee WJ, Park CY. Gemigliptin: an update of its clinical use in the management of type 2 diabetes mellitus. Diabetes Metab J. 2016; 40:339–53.
Article12. Park SE, Lee BW, Kim JH, Lee WJ, Cho JH, Jung CH, et al. Effect of gemigliptin on glycaemic variability in patients with type 2 diabetes (STABLE study). Diabetes Obes Metab. 2017; 19:892–6.
Article13. Rhee EJ, Lee WY, Min KW, Shivane VK, Sosale AR, Jang HC, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor gemigliptin compared with sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Obes Metab. 2013; 15:523–30.
Article14. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27:1487–95.
Article15. Zhu L, Tamvakopoulos C, Xie D, Dragovic J, Shen X, Fenyk-Melody JE, et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38). J Biol Chem. 2003; 278:22418–23.16. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013; 56:696–708.
Article17. Rhee EJ. Diabetes in Asians. Endocrinol Metab (Seoul). 2015; 30:263–9.
Article18. Chang AM, Smith MJ, Galecki AT, Bloem CJ, Halter JB. Impaired beta-cell function in human aging: response to nicotinic acid-induced insulin resistance. J Clin Endocrinol Metab. 2006; 91:3303–9.19. Cobelli C, Toffolo GM, Dalla Man C, Campioni M, Denti P, Caumo A, et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab. 2007; 293:E1–15.20. Szoke E, Shrayyef MZ, Messing S, Woerle HJ, van Haeften TW, Meyer C, et al. Effect of aging on glucose homeostasis: accelerated deterioration of beta-cell function in individuals with impaired glucose tolerance. Diabetes Care. 2008; 31:539–43.21. Cai X, Xia L, Pan Y, He D, Zhu H, Wei T, et al. Differential role of insulin resistance and β-cell function in the development of prediabetes and diabetes in middle-aged and elderly Chinese population. Diabetol Metab Syndr. 2019; 11:24.
Article22. Faerch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO Study. Diabetes. 2015; 64:2513–25.
Article23. Koopman ADM, Rutters F, Rauh SP, Nijpels G, Holst JJ, Beulens JW, et al. Incretin responses to oral glucose and mixed meal tests and changes in fasting glucose levels during 7 years of follow-up: the Hoorn Meal Study. PLoS One. 2018; 13:e0191114.
Article24. Pham H, Marathe CS, Phillips LK, Trahair LG, Hatzinikolas S, Huynh L, et al. Longitudinal changes in fasting and glucosestimulated GLP-1 and GIP in healthy older subjects. J Clin Endocrinol Metab. 2019; 104:6201–6.
Article25. Gallwitz B. Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne). 2019; 10:389.
Article26. Hong S, Park CY, Han KA, Chung CH, Ku BJ, Jang HC, et al. Efficacy and safety of teneligliptin, a novel dipeptidyl peptidase-4 inhibitor, in Korean patients with type 2 diabetes mellitus: a 24- week multicentre, randomized, double-blind, placebo-controlled phase III trial. Diabetes Obes Metab. 2016; 18:528–32.
Article27. Hong SM, Park CY, Hwang DM, Han KA, Lee CB, Chung CH, et al. Efficacy and safety of adding evogliptin versus sitagliptin for metformin-treated patients with type 2 diabetes: a 24-week randomized, controlled trial with open label extension. Diabetes Obes Metab. 2017; 19:654–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Emerging Safety Issues of Dipeptidyl Peptidase-4 Inhibitors and Sodium Glucose Cotransporter 2 Inhibitors: How to Interpret and Apply in Clinical Practice
- Novel Therapies for Type 2 Diabetes Mellitus
- Dipeptidyl Peptidase 4 Inhibitor, an Update
- Comparison of DPP-4 Inhibitors