Diabetes Metab J.
2022 Jan;46(1):38-48. 10.4093/dmj.2021.0045.
Regulation of Pancreatic β-Cell Mass by Gene-Environment Interaction
- Affiliations
-
- 1Division of Diabetes and Endocrinology, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan
- 2Division of Medical Chemistry, Department of Metabolism and Diseases, Kobe University Graduate School of Health Sciences, Kobe, Japan
- KMID: 2525124
- DOI: http://doi.org/10.4093/dmj.2021.0045
Abstract
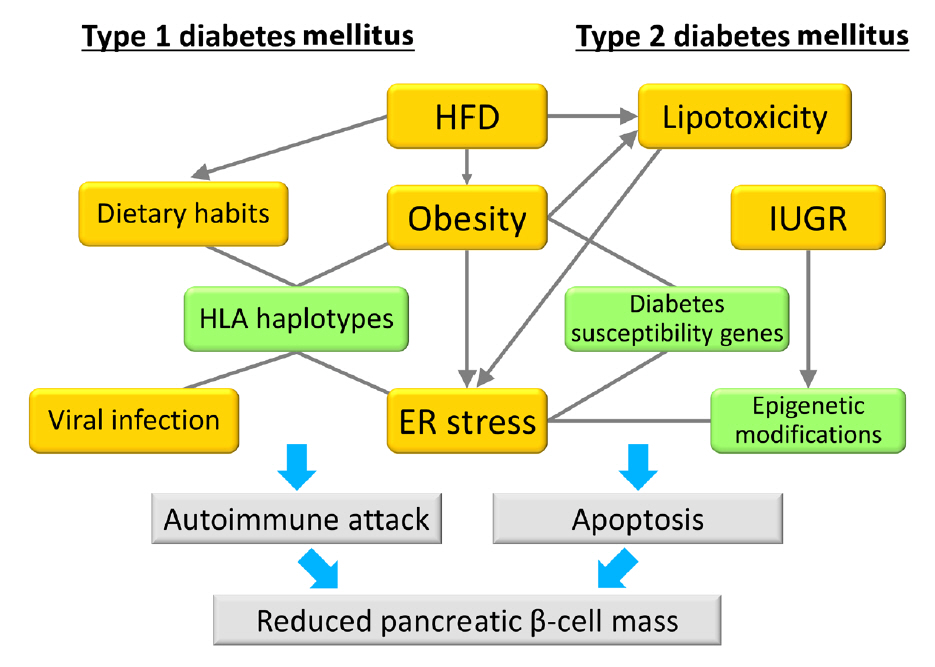
- The main pathogenic mechanism of diabetes consists of an increase in insulin resistance and a decrease in insulin secretion from pancreatic β-cells. The number of diabetic patients has been increasing dramatically worldwide, especially in Asian people whose capacity for insulin secretion is inherently lower than that of other ethnic populations. Causally, changes of environmental factors in addition to intrinsic genetic factors have been considered to have an influence on the increased prevalence of diabetes. Particular focus has been placed on “gene-environment interactions” in the development of a reduced pancreatic β-cell mass, as well as type 1 and type 2 diabetes mellitus. Changes in the intrauterine environment, such as intrauterine growth restriction, contribute to alterations of gene expression in pancreatic β-cells, ultimately resulting in the development of pancreatic β-cell failure and diabetes. As a molecular mechanism underlying the effect of the intrauterine environment, epigenetic modifications have been widely investigated. The association of diabetes susceptibility genes or dietary habits with gene-environment interactions has been reported. In this review, we provide an overview of the role of gene-environment interactions in pancreatic β-cell failure as revealed by previous reports and data from experiments.
Keyword
Figure
Cited by 1 articles
-
Protein Arginine Methyltransferases: Emerging Targets in Cardiovascular and Metabolic Disease
Yan Zhang, Shibo Wei, Eun-Ju Jin, Yunju Jo, Chang-Myung Oh, Gyu-Un Bae, Jong-Sun Kang, Dongryeol Ryu
Diabetes Metab J. 2024;48(4):487-502. doi: 10.4093/dmj.2023.0362.
Reference
-
1. International Diabetes Federation. IDF Diabetes Atlas. 9th ed. Brussels: International Diabetes Federation;2019.2. Yabe D, Seino Y. Type 2 diabetes via β-cell dysfunction in East Asian people. Lancet Diabetes Endocrinol. 2016; 4:2–3.
Article3. Yabe D, Seino Y, Fukushima M, Seino S. β Cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diab Rep. 2015; 15:602.
Article4. Cho YS, Chen CH, Hu C, Long J, Ong RT, Sim X, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in East Asians. Nat Genet. 2011; 44:67–72.5. Hashimoto N, Kido Y, Uchida T, Asahara S, Shigeyama Y, Matsuda T, et al. Ablation of PDK1 in pancreatic beta cells induces diabetes as a result of loss of beta cell mass. Nat Genet. 2006; 38:589–93.
Article6. Shigeyama Y, Kobayashi T, Kido Y, Hashimoto N, Asahara S, Matsuda T, et al. Biphasic response of pancreatic beta-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol Cell Biol. 2008; 28:2971–9.
Article7. Koyanagi M, Asahara S, Matsuda T, Hashimoto N, Shigeyama Y, Shibutani Y, et al. Ablation of TSC2 enhances insulin secretion by increasing the number of mitochondria through activation of mTORC1. PLoS One. 2011; 6:e23238.
Article8. Bartolome A, Kimura-Koyanagi M, Asahara S, Guillen C, Inoue H, Teruyama K, et al. Pancreatic β-cell failure mediated by mTORC1 hyperactivity and autophagic impairment. Diabetes. 2014; 63:2996–3008.
Article9. Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol. 2009; 5:e1000324.
Article10. Kim DH, Gutierrez-Aguilar R, Kim HJ, Woods SC, Seeley RJ. Increased adipose tissue hypoxia and capacity for angiogenesis and inflammation in young diet-sensitive C57 mice compared with diet-resistant FVB mice. Int J Obes (Lond). 2013; 37:853–60.
Article11. Eldar-Finkelman H, Schreyer SA, Shinohara MM, LeBoeuf RC, Krebs EG. Increased glycogen synthase kinase-3 activity in diabetes- and obesity-prone C57BL/6J mice. Diabetes. 1999; 48:1662–6.
Article12. Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988; 37:1163–7.
Article13. West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992; 262(6 Pt 2):R1025–32.
Article14. Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995; 44:645–51.
Article15. Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004; 53:3274–85.
Article16. Chiu KC, Cohan P, Lee NP, Chuang LM. Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diabetes Care. 2000; 23:1353–8.
Article17. Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002; 51:2170–8.18. Gerstein HC, Anand S, Yi QL, Vuksan V, Lonn E, Teo K, et al. The relationship between dysglycemia and atherosclerosis in South Asian, Chinese, and European individuals in Canada: a randomly sampled cross-sectional study. Diabetes Care. 2003; 26:144–9.19. Davis TM, Mulder H, Lokhnygina Y, Aschner P, Chuang LM, Raffo Grado CA, et al. Effect of race on the glycaemic response to sitagliptin: insights from the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS). Diabetes Obes Metab. 2018; 20:1427–34.
Article20. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013; 1281:64–91.
Article21. Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC. β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care. 2013; 36:111–7.22. Kou K, Saisho Y, Satoh S, Yamada T, Itoh H. Change in β-cell mass in Japanese nondiabetic obese individuals. J Clin Endocrinol Metab. 2013; 98:3724–30.
Article23. Roh E, Kim KM, Park KS, Kim YJ, Chun EJ, Choi SH, et al. Comparison of pancreatic volume and fat amount linked with glucose homeostasis between healthy Caucasians and Koreans. Diabetes Obes Metab. 2018; 20:2642–52.
Article24. Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008; 10 Suppl 4:32–42.25. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986; 1:1077–81.
Article26. Simeoni U, Osmond C, Garay R, Buffat C, Boubred F, Chagnaud C, et al. Leptin and insulin in young adulthood areassociated with weight in infancy. J Endocrinol. 2020; 244:249–59.27. Huang RC, Prescott SL, Godfrey KM, Davis EA. Assessment of cardiometabolic risk in children in population studies: underpinning developmental origins of health and disease mother-offspring cohort studies. J Nutr Sci. 2015; 4:e12.
Article28. Heindel JJ, Blumberg B. Environmental obesogens: mechanisms and controversies. Annu Rev Pharmacol Toxicol. 2019; 59:89–106.
Article29. Tain YL, Hsu CN. Developmental programming of the metabolic syndrome: can we reprogram with resveratrol? Int J Mol Sci. 2018; 19:2584.
Article30. Lee WC, Wu KLH, Leu S, Tain YL. Translational insights on developmental origins of metabolic syndrome: focus on fructose consumption. Biomed J. 2018; 41:96–101.
Article31. Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001; 185:93–8.
Article32. Painter RC, de Rooij SR, Bossuyt PM, de Groot E, Stok WJ, Osmond C, et al. Maternal nutrition during gestation and carotid arterial compliance in the adult offspring: the Dutch famine birth cohort. J Hypertens. 2007; 25:533–40.
Article33. Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005; 1:371–8.
Article34. Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004; 427:411–2.35. Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002; 109:194–9.
Article36. Nordman H, Jaaskelainen J, Voutilainen R. Birth size as a determinant of cardiometabolic risk factors in children. Horm Res Paediatr. 2020; 93:144–53.
Article37. Shi H, Yang X, Wu D, Wang X, Li T, Liu H, et al. Insights into infancy weight gain patterns for term small-for-gestationalage babies. Nutr J. 2018; 17:97.
Article38. Berends LM, Dearden L, Tung YC, Voshol P, Fernandez-Twinn DS, Ozanne SE. Programming of central and peripheral insulin resistance by low birthweight and postnatal catchup growth in male mice. Diabetologia. 2018; 61:2225–34.
Article39. Yoshida Y, Fuchita M, Kimura-Koyanagi M, Kanno A, Matsuda T, Asahara SI, et al. Contribution of insulin signaling to the regulation of pancreatic beta-cell mass during the catch-up growth period in a low birth weight mouse model. Diabetol Int. 2014; 5:43–52.
Article40. Inoue T, Kido Y, Asahara S, Matsuda T, Shibutani Y, Koyanagi M, et al. Effect of intrauterine undernutrition during late gestation on pancreatic beta cell mass. Biomed Res. 2009; 30:325–30.41. Stewart CP, Christian P, Schulze KJ, Arguello M, LeClerq SC, Khatry SK, et al. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J Nutr. 2011; 141:1912–7.
Article42. Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, et al. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol. 2003; 177:235–41.
Article43. Ozanne SE, Jensen CB, Tingey KJ, Storgaard H, Madsbad S, Vaag AA. Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia. 2005; 48:547–52.
Article44. McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest. 2009; 119:323–35.
Article45. Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature. 2010; 467:963–6.
Article46. Gruenert DC, Cozens AL. Inheritance of phenotype in mammalian cells: genetic vs. epigenetic mechanisms. Am J Physiol. 1991; 260(6 Pt 1):L386–94.
Article47. Takeshima H, Ushijima T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. NPJ Precis Oncol. 2019; 3:7.
Article48. Dalfra MG, Burlina S, Del Vescovo GG, Lapolla A. Genetics and epigenetics: new insight on gestational diabetes mellitus. Front Endocrinol (Lausanne). 2020; 11:602477.49. Duran Fernandez-Feijoo C, Carrasco Carrasco C, Villalmazo Francisco N, Cebria Romero J, Fernandez Lorenzo JR, Jimenez-Chillaron JC, et al. Influence of catch up growth on spatial learning and memory in a mouse model of intrauterine growth restriction. PLoS One. 2017; 12:e0177468.
Article50. Suter MA, Ma J, Vuguin PM, Hartil K, Fiallo A, Harris RA, et al. In utero exposure to a maternal high-fat diet alters the epigenetic histone code in a murine model. Am J Obstet Gynecol. 2014; 210:463.
Article51. Tozour J, Hughes F, Carrier A, Vieau D, Delahaye F. Prenatal hyperglycemia exposure and cellular stress, a sugar-coated view of early programming of metabolic diseases. Biomolecules. 2020; 10:1359.
Article52. Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest. 2008; 118:2316–24.
Article53. Sandovici I, Smith NH, Nitert MD, Ackers-Johnson M, UribeLewis S, Ito Y, et al. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci U S A. 2011; 108:5449–54.54. Einstein F, Thompson RF, Bhagat TD, Fazzari MJ, Verma A, Barzilai N, et al. Cytosine methylation dysregulation in neonates following intrauterine growth restriction. PLoS One. 2010; 5:e8887.
Article55. Xing Y, Zhang J, Wei H, Zhang H, Guan Y, Wang X, et al. Reduction of the PI3K/Akt related signaling activities in skeletal muscle tissues involves insulin resistance in intrauterine growth restriction rats with catch-up growth. PLoS One. 2019; 14:e0216665.
Article56. Dunlop K, Cedrone M, Staples JF, Regnault TR. Altered fetal skeletal muscle nutrient metabolism following an adverse in utero environment and the modulation of later life insulin sensitivity. Nutrients. 2015; 7:1202–16.
Article57. Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J Biol Chem. 2008; 283:13611–26.
Article58. Ehara T, Kamei Y, Takahashi M, Yuan X, Kanai S, Tamura E, et al. Role of DNA methylation in the regulation of lipogenic glycerol-3-phosphate acyltransferase 1 gene expression in the mouse neonatal liver. Diabetes. 2012; 61:2442–50.
Article59. Martinez D, Pentinat T, Ribo S, Daviaud C, Bloks VW, Cebria J, et al. In utero undernutrition in male mice programs liver lipid metabolism in the second-generation offspring involving altered Lxra DNA methylation. Cell Metab. 2014; 19:941–51.
Article60. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014; 383:69–82.
Article61. Incani M, Serafini C, Satta C, Perra L, Scano F, Frongia P, et al. High prevalence of diabetes-specific autoimmunity in firstdegree relatives of Sardinian patients with type 1 diabetes. Diabetes Metab Res Rev. 2017; 33:e2864.
Article62. You WP, Henneberg M. Type 1 diabetes prevalence increasing globally and regionally: the role of natural selection and life expectancy at birth. BMJ Open Diabetes Res Care. 2016; 4:e000161.
Article63. Monaghan M, Helgeson V, Wiebe D. Type 1 diabetes in young adulthood. Curr Diabetes Rev. 2015; 11:239–50.
Article64. DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med. 2006; 23:857–66.65. Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G; EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009; 373:2027–33.
Article66. Noble JA, Valdes AM. Genetics of the HLA region in the prediction of type 1 diabetes. Curr Diab Rep. 2011; 11:533–42.
Article67. Mishra R, Akerlund M, Cousminer DL, Ahlqvist E, Bradfield JP, Chesi A, et al. Genetic discrimination between LADA and childhood-onset type 1 diabetes within the MHC. Diabetes Care. 2020; 43:418–25.
Article68. Buzzetti R, Prudente S, Copetti M, Dauriz M, Zampetti S, Garofolo M, et al. Clinical worthlessness of genetic prediction of common forms of diabetes mellitus and related chronic complications: a position statement of the Italian Society of Diabetology. Nutr Metab Cardiovasc Dis. 2017; 27:99–114.
Article69. NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016; 387:1377–96.70. Asahara SI, Miura H, Ogawa W, Tamori Y. Sex difference in the association of obesity with personal or social background among urban residents in Japan. PLoS One. 2020; 15:e0242105.
Article71. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017; 390:2627–42.72. De Keukelaere M, Fieuws S, Reynaert N, Vandoorne E, Kerckhove KV, Asscherickx W, et al. Evolution of body mass index in children with type 1 diabetes mellitus. Eur J Pediatr. 2018; 177:1661–6.
Article73. Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ, et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev. 2018; 39:629–63.
Article74. Patterson CC, Harjutsalo V, Rosenbauer J, Neu A, Cinek O, Skrivarhaug T, et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia. 2019; 62:408–17.
Article75. Public Health Report: Overweight and obesity in Norway. Available from: https://www.fhi.no/en/op/hin/health-disease/overweight-and-obesity-in-norway--- (updated 2017 Nov 3).76. Fellinger P, Fuchs D, Wolf P, Heinze G, Luger A, Krebs M, et al. Overweight and obesity in type 1 diabetes equal those of the general population. Wien Klin Wochenschr. 2019; 131:55–60.
Article77. Ferrara CT, Geyer SM, Liu YF, Evans-Molina C, Libman IM, Besser R, et al. Excess BMI in childhood: a modifiable risk factor for type 1 diabetes development? Diabetes Care. 2017; 40:698–701.
Article78. Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017; 127:1–4.
Article79. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016; 387:2340–8.
Article80. Nekoua MP, Bertin A, Sane F, Alidjinou EK, Lobert D, Trauet J, et al. Pancreatic beta cells persistently infected with coxsackievirus B4 are targets of NK cell-mediated cytolytic activity. Cell Mol Life Sci. 2020; 77:179–94.
Article81. Lamb MM, Miller M, Seifert JA, Frederiksen B, Kroehl M, Rewers M, et al. The effect of childhood cow’s milk intake and HLA-DR genotype on risk of islet autoimmunity and type 1 diabetes: the Diabetes Autoimmunity Study in the Young. Pediatr Diabetes. 2015; 16:31–8.
Article82. Lempainen J, Tauriainen S, Vaarala O, Makela M, Honkanen H, Marttila J, et al. Interaction of enterovirus infection and cow’s milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diabetes Metab Res Rev. 2012; 28:177–85.
Article83. Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003; 290:1721–8.
Article84. Uusitalo U, Lee HS, Andren Aronsson C, Vehik K, Yang J, Hummel S, et al. Early infant diet and islet autoimmunity in the TEDDY study. Diabetes Care. 2018; 41:522–30.85. Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus: why the β cells? Nat Rev Endocrinol. 2016; 12:263–73.
Article86. Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013; 56:234–41.
Article87. Thomaidou S, Zaldumbide A, Roep BO. Islet stress, degradation and autoimmunity. Diabetes Obes Metab. 2018; 20(Suppl 2):88–94.
Article88. Kracht MJ, van Lummel M, Nikolic T, Joosten AM, Laban S, van der Slik AR, et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat Med. 2017; 23:501–7.
Article89. Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, et al. Variants in KCNQ1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008; 40:1092–7.
Article90. Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet. 2008; 40:1098–102.
Article91. Hu C, Wang C, Zhang R, Ma X, Wang J, Lu J, et al. Variations in KCNQ1 are associated with type 2 diabetes and beta cell function in a Chinese population. Diabetologia. 2009; 52:1322–5.
Article92. Jonsson A, Isomaa B, Tuomi T, Taneera J, Salehi A, Nilsson P, et al. A variant in the KCNQ1 gene predicts future type 2 diabetes and mediates impaired insulin secretion. Diabetes. 2009; 58:2409–13.93. Tan JT, Nurbaya S, Gardner D, Ye S, Tai ES, Ng DP. Genetic variation in KCNQ1 associates with fasting glucose and betacell function: a study of 3,734 subjects comprising three ethnicities living in Singapore. Diabetes. 2009; 58:1445–9.94. Rosengren AH, Braun M, Mahdi T, Andersson SA, Travers ME, Shigeto M, et al. Reduced insulin exocytosis in human pancreatic β-cells with gene variants linked to type 2 diabetes. Diabetes. 2012; 61:1726–33.
Article95. Asahara S, Etoh H, Inoue H, Teruyama K, Shibutani Y, Ihara Y, et al. Paternal allelic mutation at the Kcnq1 locus reduces pancreatic β-cell mass by epigenetic modification of Cdkn1c. Proc Natl Acad Sci U S A. 2015; 112:8332–7.96. Matsuda T, Kido Y, Asahara S, Kaisho T, Tanaka T, Hashimoto N, et al. Ablation of C/EBPbeta alleviates ER stress and pancreatic beta cell failure through the GRP78 chaperone in mice. J Clin Invest. 2010; 120:115–26.97. Matsuda T, Takahashi H, Mieda Y, Shimizu S, Kawamoto T, Matsuura Y, et al. Regulation of pancreatic β cell mass by cross-interaction between CCAAT enhancer binding protein β induced by endoplasmic reticulum stress and AMP-activated protein kinase activity. PLoS One. 2015; 10:e0130757.
Article98. Deval C, Chaveroux C, Maurin AC, Cherasse Y, Parry L, Carraro V, et al. Amino acid limitation regulates the expression of genes involved in several specific biological processes through GCN2-dependent and GCN2-independent pathways. FEBS J. 2009; 276:707–18.
Article99. Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002; 22:6681–8.
Article100. Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007; 5:103–14.101. Miyake K, Yang W, Hara K, Yasuda K, Horikawa Y, Osawa H, et al. Construction of a prediction model for type 2 diabetes mellitus in the Japanese population based on 11 genes with strong evidence of the association. J Hum Genet. 2009; 54:236–41.
Article102. Kanno A, Asahara SI, Furubayashi A, Masuda K, Yoshitomi R, Suzuki E, et al. GCN2 regulates pancreatic β cell mass by sensing intracellular amino acid levels. JCI Insight. 2020; 5:e128820.
Article103. Kanno A, Asahara SI, Masuda K, Matsuda T, Kimura-Koyanagi M, Seino S, et al. Compensatory hyperinsulinemia in high-fat diet-induced obese mice is associated with enhanced insulin translation in islets. Biochem Biophys Res Commun. 2015; 458:681–6.
Article104. Zhou T, Kim TW, Chong CN, Tan L, Amin S, Sadat Badieyan Z, et al. A hPSC-based platform to discover gene-environment interactions that impact human β-cell and dopamine neuron survival. Nat Commun. 2018; 9:4815.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids
- Interpretation of the hygiene and microflora hypothesis for allergic diseases through epigenetic epidemiology
- Suicide : Gene-Environment Interaction
- B-cell translocation gene 2 positively regulates GLP-1-stimulated insulin secretion via induction of PDX-1 in pancreatic beta-cells
- Gene-Environment Interactions Should be Considered in Future Studies to Understand the Association Between Prenatal Folate Supplementation and Asthma Development