Kosin Med J.
2021 Dec;36(2):109-115. 10.7180/kmj.2021.36.2.109.
The Natural Course of Total Kidney Volume in Patients with Autosomal Dominant Polycystic Kidney Disease undergoing Hemodialysis
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan, Republic of Korea
- 2Department of Radiology, Kosin University College of Medicine, Busan, Republic of Korea
- 3Department of Internal Medicine, Bong Seng Hospital, Busan, Republic of Korea
- KMID: 2524642
- DOI: http://doi.org/10.7180/kmj.2021.36.2.109
Abstract
Objectives
The natural course of native kidneys after hemodialysis initiation in patients with autosomal dominant polycystic kidney disease (ADPKD) remains poorly understood.
Methods
We measured the total volumes of native kidneys in 12 patients who had at least one enhanced computed tomography (CT) image both before and after initiation of hemodialysis (group 1) and in 18 patients who had no image before dialysis but more than two images after dialysis (group 2). In patients with images, the last image was used for analysis only after dialysis.
Results
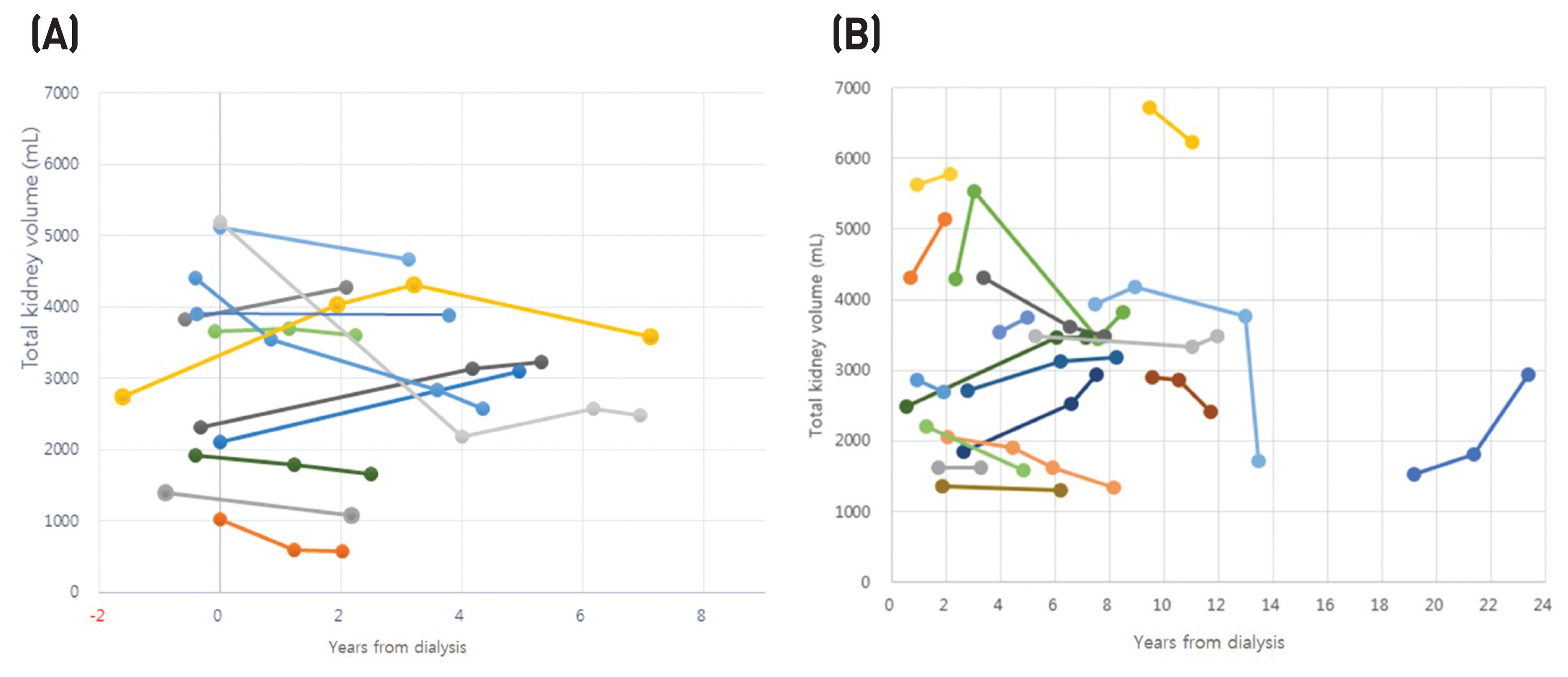
The mean total kidney volume (TKV) (± SD) before hemodialysis initiation was 3132 ± 1413 mL and the mean TKV of the last image was 3047 ± 1323 mL in group 1. The mean TKV change rate (%) was - 5.2 ± 27.4% (P > 0.05) during follow-up of 3.9 ± 1.9 years in group 1. The mean TKV change rate was 2.8 ± 34.4% (P > 0.05) in group 2. The follow-up period after dialysis initiation ranged from 4.2 ± 4.7 to 8.0 ± 5.2 years.
Conclusions
The results suggest that the TKV of native polycystic kidneys decreases substantially after hemodialysis initiation. This reduction occurs mainly during the early post-hemodialysis period and followed by a slow enlargement of TKV.
Figure
Reference
-
1. Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993; 329:332–42.
Article2. Harris PC, Torres VE. Polycystic Kidney Disease, Autosomal Dominant. Adam MP, Ardinger HH, Pagon RA, editors. GeneReviews. Seattle (WA): University of Washington;1993–2020. p. 1–44.3. Chebib FT, Prieto M, Jung Y, Irazabal MV, Kremers WK, Dean PG, et al. Native Nephrectomy in Renal Transplant Recipients with Autosomal Dominant Polycystic Kidney Disease. Transplant Direct. 2015; 1:e43.4. Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006; 354:2122–30.
Article5. Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015; 26:160–72.
Article6. Ishikawa I, Saito Y. Volume changes in autosomal dominant polycystic kidneys after the initiation of hemodialysis. Nephron. 1993; 65:649–50.
Article7. Ishikawa I, Tateishi K, Kitada H, Shinoda A. Regression of adult type polycystic kidneys during chronic intermittent hemodialysis. Is it a universal phenomenon? Nephron. 1984; 36:147.
Article8. Thaysen JH, Thomsen HS, Sass A, Kristensen JK. Volume changes in polycystic kidneys during chronic dialysis and after renal transplantation. Acta Med Scand. 1985; 217:197–204.
Article9. Jung Y, Irazabal MV, Chebib FT, Harris PC, Dean PG, Prieto M, et al. Volume regression of native polycystic kidneys after renal transplantation. Nephrol Dial Transplant. 2016; 31:73–9.
Article10. Yamamoto T, Watarai Y, Kobayashi T, Matsuda Y, Tsujita M, Hiramitsu T, et al. Kidney volume changes in patients with autosomal dominant polycystic kidney disease after renal transplantation. Transplantation. 2012; 93:794–8.
Article11. Grantham JJ. Acquired cystic kidney disease. Kidney Int. 1991; 40:143–52.
Article12. Ishikawa I. Acquired cystic disease: mechanisms and manifestations. Semin Nephrol. 1991; 11:671–84.13. Herrera GA. C-erb B-2 amplification in cystic renal disease. Kidney Int. 1991; 40:509–13.
Article14. Konda R, Sato H, Hatafuku F, Nozawa T, Ioritani N, Fujioka T. Expression of hepatocyte growth factor and its receptor C-met in acquired renal cystic disease associated with renal cell carcinoma. J Urol. 2004; 171:2166–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Renal Cell Carcinoma in Autosomal Dominant Polycystic Kidney Disease Hemodialyzed
- Management of autosomal dominant polycystic kidney disease in the era of disease-modifying treatment options
- Segmental Cystic Disease of the Kidney: A Case Report
- Autosomal Dominant Polycystic Kidney Disease: 2009 Update for Internists
- Autosomal Dominant Polycystic Kidney Desease Coexisting with Renal Dysplasia. First Case Described and Followed Since Prenatal Period