Cancer Res Treat.
2022 Jan;54(1):301-313. 10.4143/crt.2020.1371.
Role of Roflumilast Combined with ESHAP Chemotherapy in Relapsed/Refractory Patients with Diffuse Large B-Cell Lymphoma
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Internal Medicine, Biochemical Research Institution, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 2Department of Biological Sciences, Pusan National University, Busan, Korea
- KMID: 2524611
- DOI: http://doi.org/10.4143/crt.2020.1371
Abstract
- Purpose
There are unmet needs associated with the current treatment strategies for relapsed/refractory diffuse large B-cell lymphoma (DLBCL) due to the poor treatment outcomes of these strategies. Roflumilast, a selective phosphodiesterase-4 inhibitor used for treating chronic obstructive pulmonary disease, is effective against B-cell malignancy via phosphoinositide 3-kinase (PI3K)–activity suppression. We analyzed the effects of roflumilast combined with ESHAP (etoposide, cisplatin, methylprednisolone, and cytarabine) chemotherapy in experimental and clinical settings.
Materials and Methods
An in vitro study using lymphoma cell lines and a pilot study on relapsed/refractory DLBCL patients were conducted to investigate the effects and mechanism of the combination of roflumilast and chemotherapy. The complete response (CR), overall response rate (ORR), and 1-year progression-free survival (PFS) were analyzed.
Results
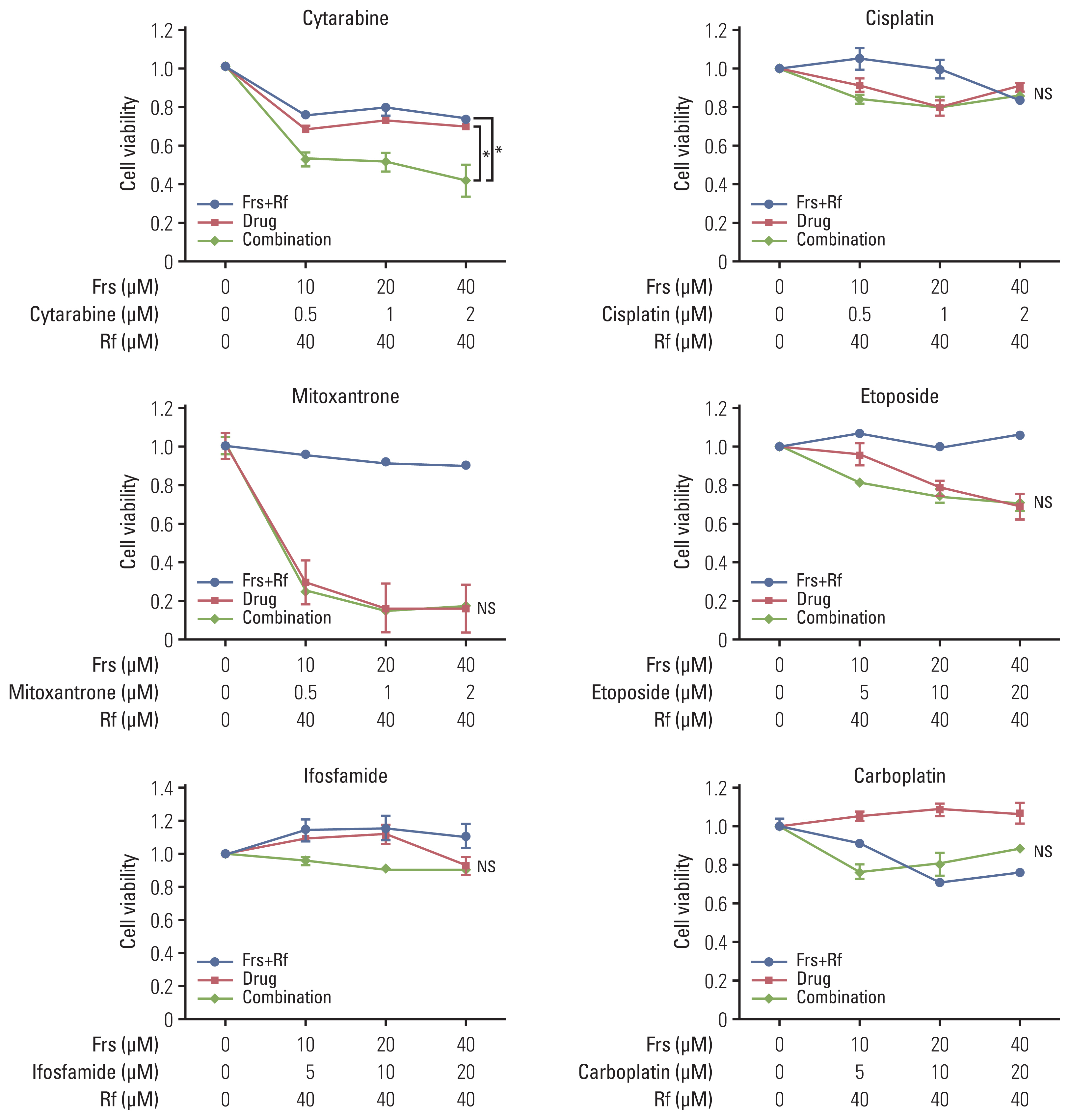
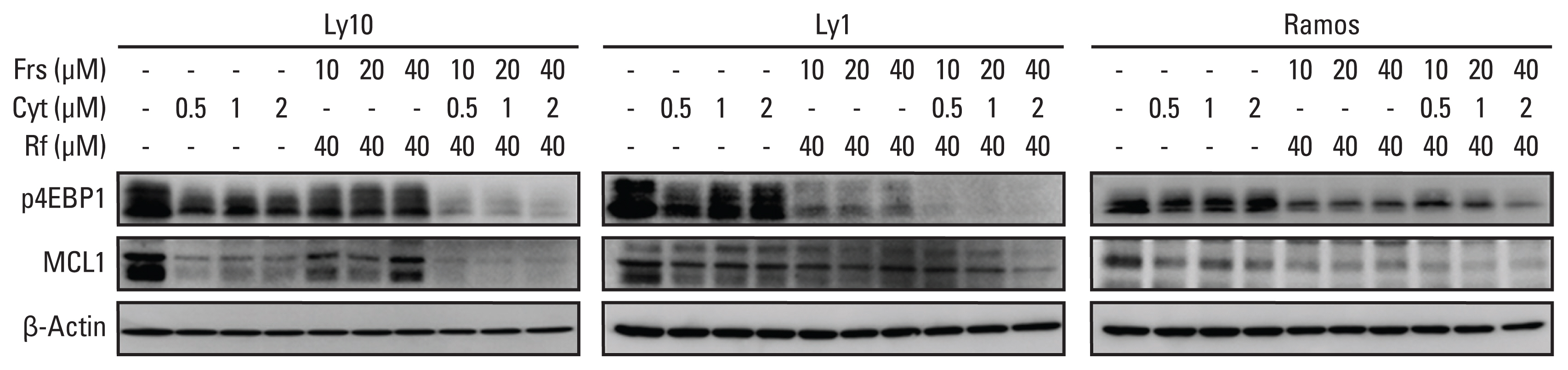
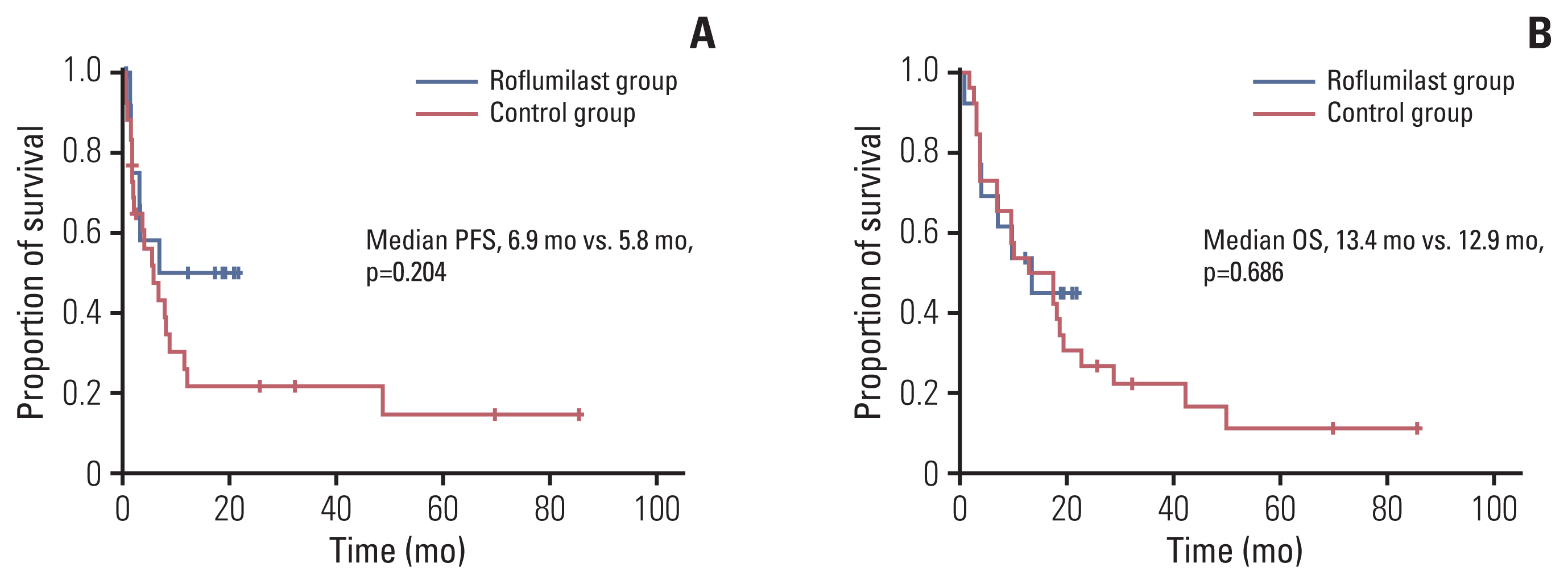
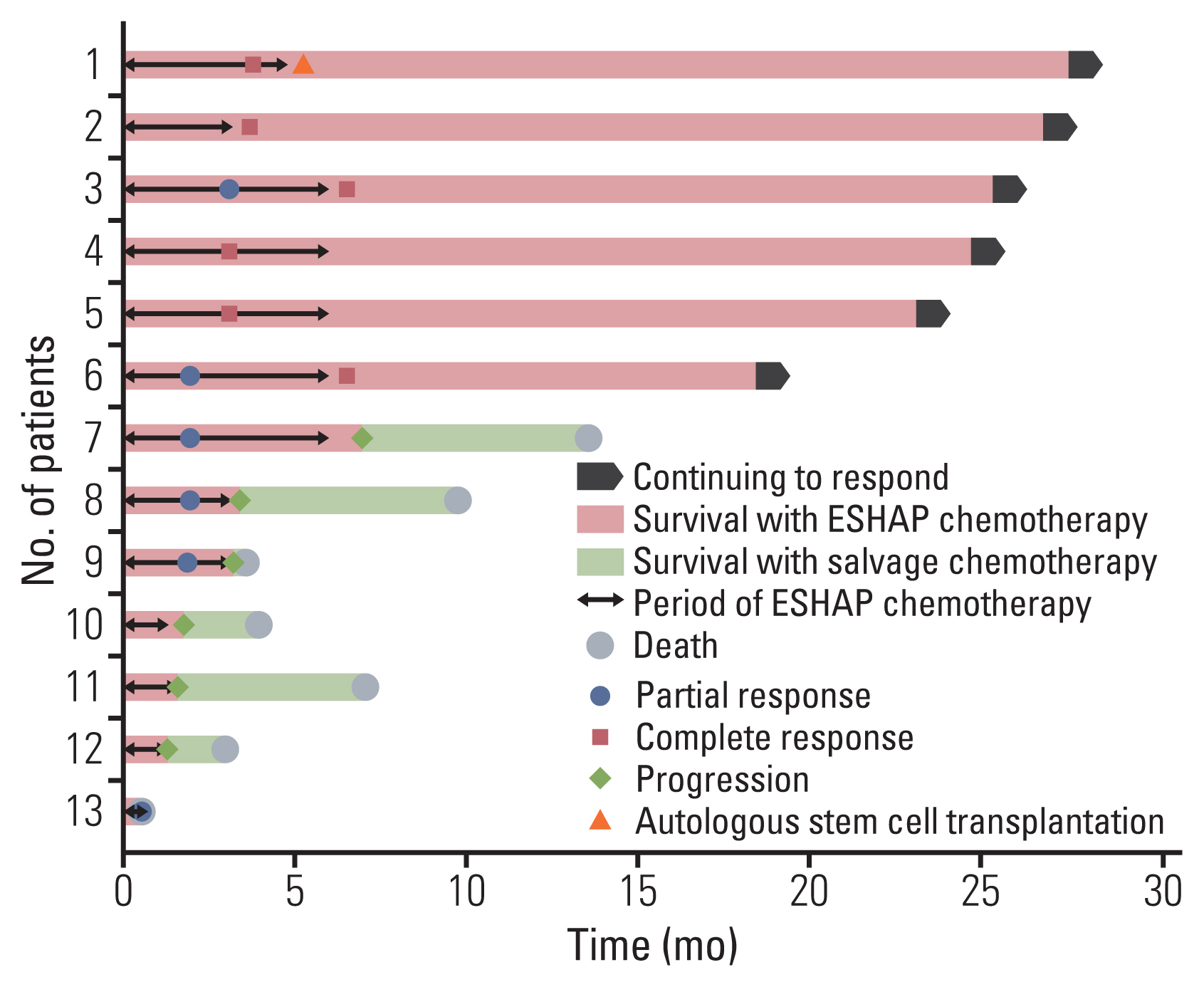
We found that roflumilast is efficient when combined with other chemotherapy drugs, especially cytarabine. Synergistic effects between these two drugs influence the translation of mammalian target of rapamycin and myeloid cell leukemia 1, resulting in apoptosis and inhibition of B-cell lymphoma proliferation. In clinical setting, the roflumilast group showed better rates of CR (46.2% vs. 34.6%), ORR (76.9% vs. 53.8%), and 1-year PFS (50.0% vs. 25.9%) compared with the control group, though not statistically significant. The roflumilast group showed a higher incidence of asthenia and gastrointestinal adverse events. However, grade 3 or 4 adverse events were similar in both groups.
Conclusion
We found that roflumilast, when combined with ESHAP chemotherapy, for relapsed/refractory DLBCL was clinically active and well tolerated. This combined treatment was able to suppress PI3K activity, which is correlated with the degree of clinical response.
Keyword
Figure
Reference
-
References
1. Lee H, Park HJ, Park EH, Ju HY, Oh CM, Kong HJ, et al. Nationwide statistical analysis of lymphoid malignancies in Korea. Cancer Res Treat. 2018; 50:222–38.
Article2. Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013; 87:146–71.
Article3. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–42.
Article4. Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn JY, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1987; 316:1493–8.
Article5. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017; 130:1800–8.
Article6. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28:4184–90.
Article7. National Comprehensive Cancer Network. NCCN guidelines for diffuse large B-cell lymphoma. Plymouth Meeting, PA: National Comprehensive Cancer Network;2020.8. Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2016; 11:81–90.
Article9. Smith PG, Wang F, Wilkinson KN, Savage KJ, Klein U, Neuberg DS, et al. The phosphodiesterase PDE4B limits cAMP-associated PI3K/AKT-dependent apoptosis in diffuse large B-cell lymphoma. Blood. 2005; 105:308–16.
Article10. Suhasini AN, Wang L, Holder KN, Lin AP, Bhatnagar H, Kim SW, et al. A phosphodiesterase 4B-dependent interplay between tumor cells and the microenvironment regulates angiogenesis in B-cell lymphoma. Leukemia. 2016; 30:617–26.
Article11. Bosnjak M, Ristic B, Arsikin K, Mircic A, Suzin-Zivkovic V, Perovic V, et al. Inhibition of mTOR-dependent autophagy sensitizes leukemic cells to cytarabine-induced apoptotic death. PLoS One. 2014; 9:e94374.
Article12. Kim SW, Rai D, Aguiar RC. Gene set enrichment analysis unveils the mechanism for the phosphodiesterase 4B control of glucocorticoid response in B-cell lymphoma. Clin Cancer Res. 2011; 17:6723–32.
Article13. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Primary analysis of juliet: a global, pivotal, phase 2 trial of CTL019 in adult patients with relapsed or refractory diffuse large B-cell lymphoma. Blood. 2017; 130(Suppl 1):577.14. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020; 38:155–65.
Article15. Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016; 127:1410–6.
Article16. Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv. 2002; 2:168–84.
Article17. Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006; 58:488–520.
Article18. Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002; 8:68–74.
Article19. Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004; 350:1828–37.
Article20. Zhao S, Dong X, Shen W, Ye Z, Xiang R. Machine learning-based classification of diffuse large B-cell lymphoma patients by eight gene expression profiles. Cancer Med. 2016; 5:837–52.
Article21. Zhao KH, Zhang C, Bai Y, Li Y, Kang X, Chen JX, et al. Antiglioma effects of cytarabine on leptomeningeal metastasis of high-grade glioma by targeting the PI3K/Akt/mTOR pathway. Drug Des Devel Ther. 2017; 11:1905–15.
Article22. Galmarini CM, Jordheim L, Dumontet C. Pyrimidine nucleoside analogs in cancer treatment. Expert Rev Anticancer Ther. 2003; 3:717–28.
Article23. Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP: an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994; 12:1169–76.24. Ezzat AA, Khalifa F, Berry J, Khan B, Raja MA, Abdel-Warith A. E-SHAP: an effective treatment in selected patients with relapsed non-Hodgkin’s lymphoma. Ann Oncol. 1994; 5:453–6.
Article25. Choi CW, Paek CW, Seo JH, Kim BS, Shin SW, Kim YH, et al. ESHAP salvage therapy for relapsed or refractory non-Hodgkin’s lymphoma. J Korean Med Sci. 2002; 17:621–4.
Article26. Martin A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, et al. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008; 93:1829–36.
Article27. Kim DU, Kwak B, Kim SW. Phosphodiesterase 4B is an effective therapeutic target in colorectal cancer. Biochem Biophys Res Commun. 2019; 508:825–31.
Article28. Domvri K, Zarogoulidis K, Zogas N, Zarogoulidis P, Petanidis S, Porpodis K, et al. Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer. J Cancer. 2017; 8:3648–56.
Article29. Kelly K, Mejia A, Suhasini AN, Lin AP, Kuhn J, Karnad AB, et al. Safety and pharmacodynamics of the PDE4 inhibitor roflumilast in advanced B-cell malignancies. Clin Cancer Res. 2017; 23:1186–92.
Article30. Cooney JD, Aguiar RC. Phosphodiesterase 4 inhibitors have wide-ranging activity in B-cell malignancies. Blood. 2016; 128:2886–90.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rituximab and ESHAP as Second-line Therapy for Relapsed or Primary Refractory Diffuse Large B Cell Lymphoma: The Experience of a Single Center in Korea
- ESHAP Salvage Therapy for Relapsed or Refractory Non-Hodgkin's Lymphoma
- A Case of Solitary Relapsed Diffuse Large B-cell Lymphoma of the Gallbladder
- ESHAP Salvage Therapy for Refractory and Relapsed Non-Hodgkins Lymphoma: A Single Center Experience
- Chimeric Antigen Receptor T-Cell Therapy for Diffuse Large B-Cell Lymphoma