Cancer Res Treat.
2022 Jan;54(1):277-293. 10.4143/crt.2021.320.
Enrichment of Wee1/CDC2 and NF-κB Signaling Pathway Constituents Mutually Contributes to CDDP Resistance in Human Osteosarcoma
- Affiliations
-
- 1Derpartment of Orthopedics, Shaoguan First People’s Hospital Affiliated to Southern Medical University, Guangdong, China
- 2Orthopedics Center, Zhujiang Hospital of Southern Medical University, Guangzhou, China
- 3Department of Orthopaedics, The Affiliated Yuebei People’s Hospital of Shantou University Medical College, Shaoguan, Guangdong, China
- 4The Second School of Clinical Medicine, Southern Medical University, Guangzhou, China
- 5Department of Pathology, Guangdong Provincal People’s Hospital & Guangdong, Academy of Medical Sciences, Guangzhou, China
- 6Guangzhou Laboratory (Guangzhou International Bio Island), Guangzhou, China
- 7University of Chinese Academy of Social Sciences (Graduate School), Guangzhou, China
- KMID: 2524609
- DOI: http://doi.org/10.4143/crt.2021.320
Abstract
- Purpose
Osteosarcoma (OS) universally exhibits heterogeneity and cisplatin (CDDP) resistance. Although the Wee1/CDC2 and nuclear factor кB (NF-κB) pathways were reported to show abnormal activation in some tumor cells with CDDP resistance, whether there is any concrete connection is currently unclear. We explored it in human OS cells.
Materials and Methods
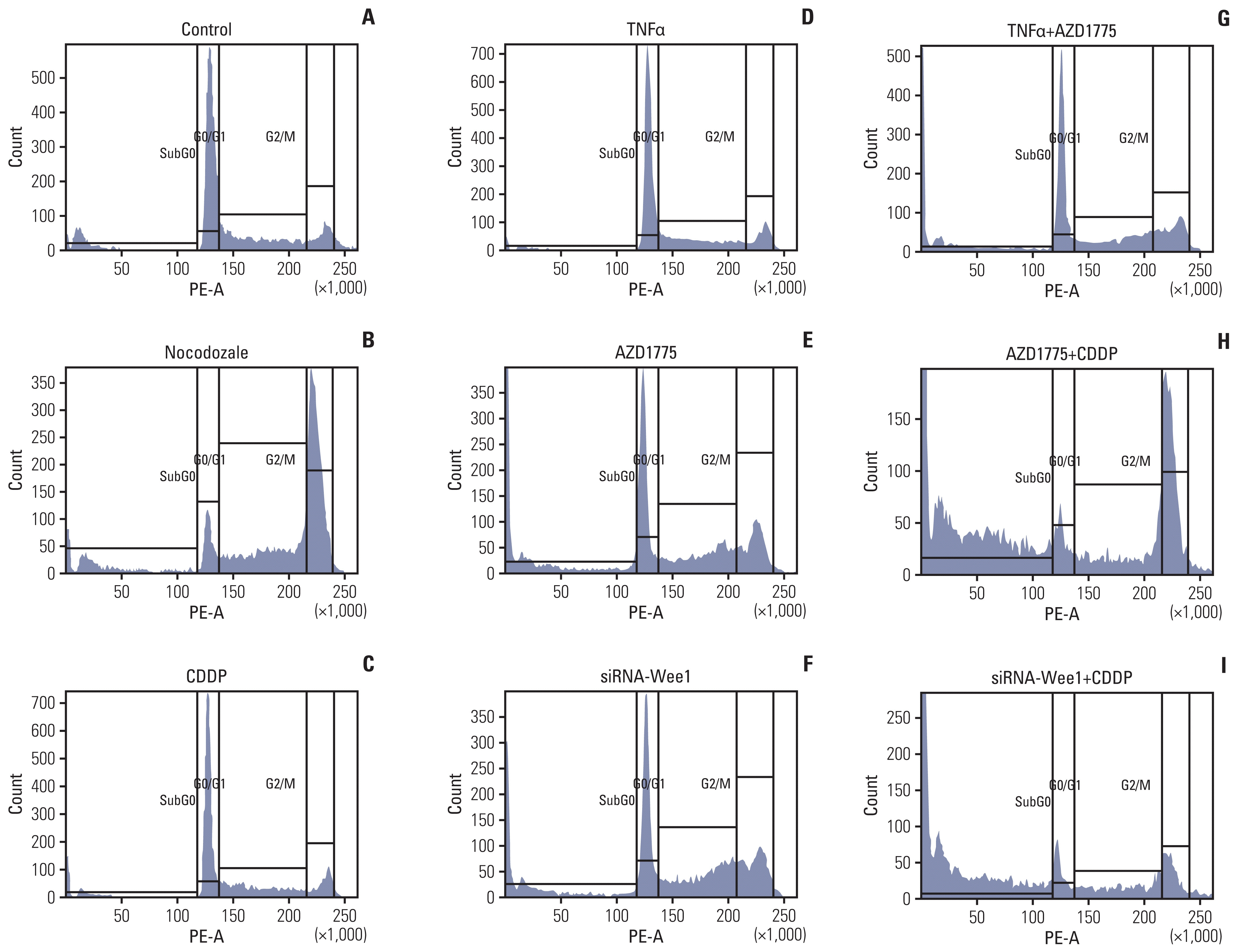
Multiple OS cell lines were exposed to a Wee1 inhibitor (AZD1775) and CDDP to assess the half-maximal inhibitory concentration values. Western blot, coimmunoprecipitation, confocal immunofluorescence, cell cycle, and Cell Counting Kit-8assays were performed to explore the connection between the Wee1/CDC2 and NF-κB pathways and their subsequent physiological contribution to CDDP resistance. Finally, CDDP-resistant PDX-OS xenograft models were established to confirm that AZD1775 restores the antitumor effects of CDDP.
Results
A sensitivity hierarchy of OS cells to CDDP and AZD1775 exists. In the highly CDDP-tolerant cell lines, Wee1 and RelA were physically crosslinked, which resulted in increased abundance of phosphorylated CDC2 (Y15) and RelA (S536) and consequent modulation of cell cycle progression, survival, and proliferation. Wee1 inhibition restored the effects of CDDP on these processes in CDDP-resistant OS cells. In addition, animal experiments with CDDP-resistant PDX-OS cells showed that AZD1775 combined with CDDP not only restored CDDP efficacy but also amplified AZD1775 in inhibiting tumor growth and prolonged the median survival of the mice.
Conclusion
Simultaneous enrichment of molecules in the Wee1/CDC2 and NF-κB pathways and their consequent coactivation is a new molecular mechanism of CDDP resistance in OS cells. OS with this molecular signature may respond well to Wee1 inhibition as an alternative treatment strategy.
Keyword
Figure
Reference
-
References
1. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015; 33:3029–35.
Article2. Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol. 2019; 53:148–58.
Article3. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018; 15:81–94.
Article4. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014; 740:364–78.
Article5. Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005; 5:749–59.6. Wang Y, Fu Q, Zhao W. Tetramethylpyrazine inhibits osteosarcoma cell proliferation via downregulation of NF-kappaB in vitro and in vivo. Mol Med Rep. 2013; 8:984–8.7. Gong Y, Zack TI, Morris LG, Lin K, Hukkelhoven E, Raheja R, et al. Pan-cancer genetic analysis identifies PARK2 as a master regulator of G1/S cyclins. Nat Genet. 2014; 46:588–94.
Article8. Miranda MA, Marcato PD, Mondal A, Chowdhury N, Gebeyehu A, Surapaneni SK, et al. Cytotoxic and chemosensitizing effects of glycoalkaloidic extract on 2D and 3D models using RT4 and patient derived xenografts bladder cancer cells. Mater Sci Eng C Mater Biol Appl. 2021; 119:111460.
Article9. Conde de la Rosa L, Schoemaker MH, Vrenken TE, Buist-Homan M, Havinga R, Jansen PL, et al. Superoxide anions and hydrogen peroxide induce hepatocyte death by different mechanisms: involvement of JNK and ERK MAP kinases. J Hepatol. 2006; 44:918–29.
Article10. Nam AR, Jin MH, Bang JH, Oh KS, Seo HR, Oh DY, et al. Inhibition of ATR increases the sensitivity to WEE1 inhibitor in biliary tract cancer. Cancer Res Treat. 2020; 52:945–56.
Article11. Yan M, Ni J, Song D, Ding M, Huang J. Activation of unfolded protein response protects osteosarcoma cells from cisplatin-induced apoptosis through NF-kappaB pathway. Int J Clin Exp Pathol. 2015; 8:10204–15.12. Moiseeva TN, Qian C, Sugitani N, Osmanbeyoglu HU, Bakkenist CJ. WEE1 kinase inhibitor AZD1775 induces CDK1 kinase-dependent origin firing in unperturbed G1- and S-phase cells. Proc Natl Acad Sci U S A. 2019; 116:23891–3.
Article13. Geenen JJ, Schellens JH. Molecular pathways: targeting the protein kinase Wee1 in cancer. Clin Cancer Res. 2017; 23:4540–4.
Article14. Kuijjer ML, Hogendoorn PC, Cleton-Jansen AM. Genome-wide analyses on high-grade osteosarcoma: making sense of a genomically most unstable tumor. Int J Cancer. 2013; 133:2512–21.
Article15. Castillo-Tandazo W, Mutsaers AJ, Walkley CR. Osteosarcoma in the post genome era: preclinical models and approaches to identify tractable therapeutic targets. Curr Osteoporos Rep. 2019; 17:343–52.
Article16. Lu W, Chao T, Ruiqi C, Juan S, Zhihong L. Patient-derived xenograft models in musculoskeletal malignancies. J Transl Med. 2018; 16:107.
Article17. Li W, Zhang X, Xi X, Li Y, Quan H, Liu S, et al. PLK2 modulation of enriched TAp73 affects osteogenic differentiation and prognosis in human osteosarcoma. Cancer Med. 2020; 9:4371–85.
Article18. Lu H, Yang X, Duggal P, Allen CT, Yan B, Cohen J, et al. TNF-alpha promotes c-REL/DeltaNp63alpha interaction and TAp73 dissociation from key genes that mediate growth arrest and apoptosis in head and neck cancer. Cancer Res. 2011; 71:6867–77.19. Blattmann C, Thiemann M, Stenzinger A, Roth EK, Dittmar A, Witt H, et al. Establishment of a patient-derived orthotopic osteosarcoma mouse model. J Transl Med. 2015; 13:136.
Article20. Hu ZB, Liao XH, Xu ZY, Yang X, Dong C, Jin AM, et al. PLK2 phosphorylates and inhibits enriched TAp73 in human osteosarcoma cells. Cancer Med. 2016; 5:74–87.21. Cho JG, Choi JS, Lee JH, Cho MG, Kim HS, Noh HD, et al. MED28 over-expression shortens the cell cycle and induces genomic instability. Int J Mol Sci. 2019; 20:1746.
Article22. Duan Z, Gao Y, Shen J, Choy E, Cote G, Harmon D, et al. miR-15b modulates multidrug resistance in human osteosarcoma in vitro and in vivo. Mol Oncol. 2017; 11:151–66.23. Lin X, Chen D, Zhang C, Zhang X, Li Z, Dong B, et al. Augmented antitumor activity by olaparib plus AZD1775 in gastric cancer through disrupting DNA damage repair pathways and DNA damage checkpoint. J Exp Clin Cancer Res. 2018; 37:129.
Article24. Woo XY, Giordano J, Srivastava A, Zhao ZM, Lloyd MW, de Bruijn R, et al. Conservation of copy number profiles during engraftment and passaging of patient-derived cancer xenografts. Nat Genet. 2021; 53:86–99.25. Teng S, Li YE, Yang M, Qi R, Huang Y, Wang Q, et al. Tissue-specific transcription reprogramming promotes liver metastasis of colorectal cancer. Cell Res. 2020; 30:34–49.
Article26. Stewart E, Federico SM, Chen X, Shelat AA, Bradley C, Gordon B, et al. Orthotopic patient-derived xenografts of paediatric solid tumours. Nature. 2017; 549:96–100.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Porcine parvovirus nonstructural protein NS1 activates NF-κB and it involves TLR2 signaling pathway
- Activation of CpG-ODN-Induced TLR9 Signaling Inhibited by Interleukin-37 in U937 Human Macrophages
- Kaempferol Regulates the Expression of Airway MUC5AC Mucin Gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 Signaling Pathways