Cancer Res Treat.
2022 Jan;54(1):218-225. 10.4143/crt.2020.1373.
Risk Factors and Patterns of Locoregional Recurrence after Radical Nephrectomy for Locally Advanced Renal Cell Carcinoma
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Urology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2524602
- DOI: http://doi.org/10.4143/crt.2020.1373
Abstract
- Purpose
We aimed to investigate the risk factors and patterns of locoregional recurrence (LRR) after radical nephrectomy (RN) in patients with locally advanced renal cell carcinoma (RCC).
Materials and Methods
We retrospectively analyzed 245 patients who underwent RN for non-metastatic pT3-4 RCC from January 2006 to January 2016. We analyzed the risk factors associated with poor locoregional control using Cox regression. Anatomical mapping was performed on reference computed tomography scans showing intact kidneys.
Results
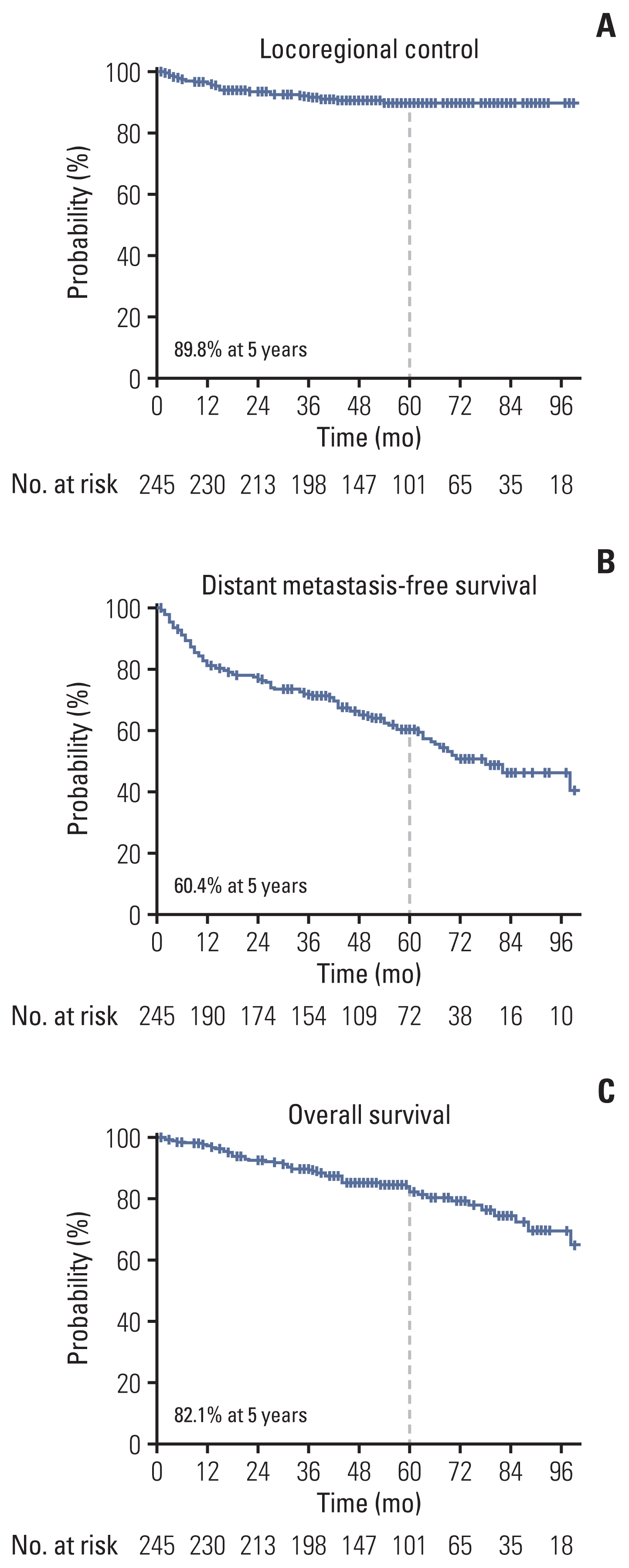
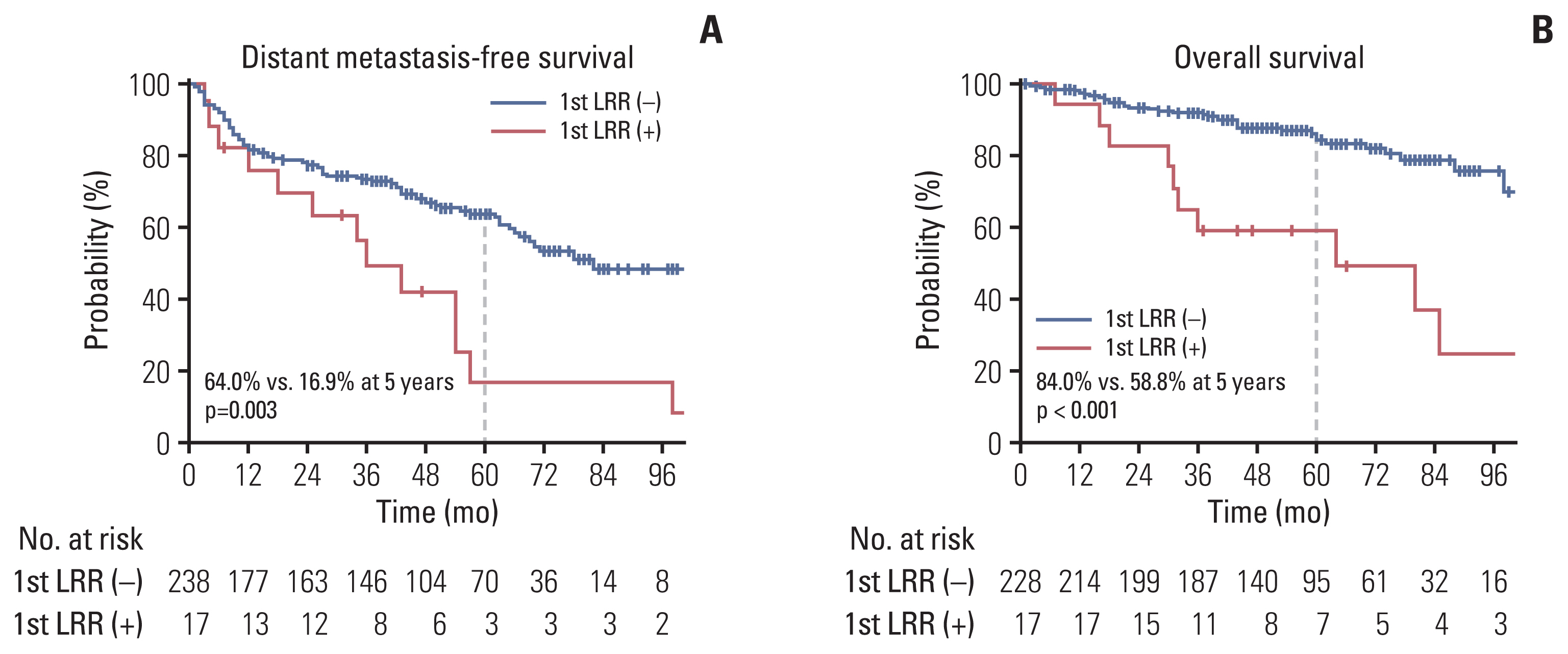
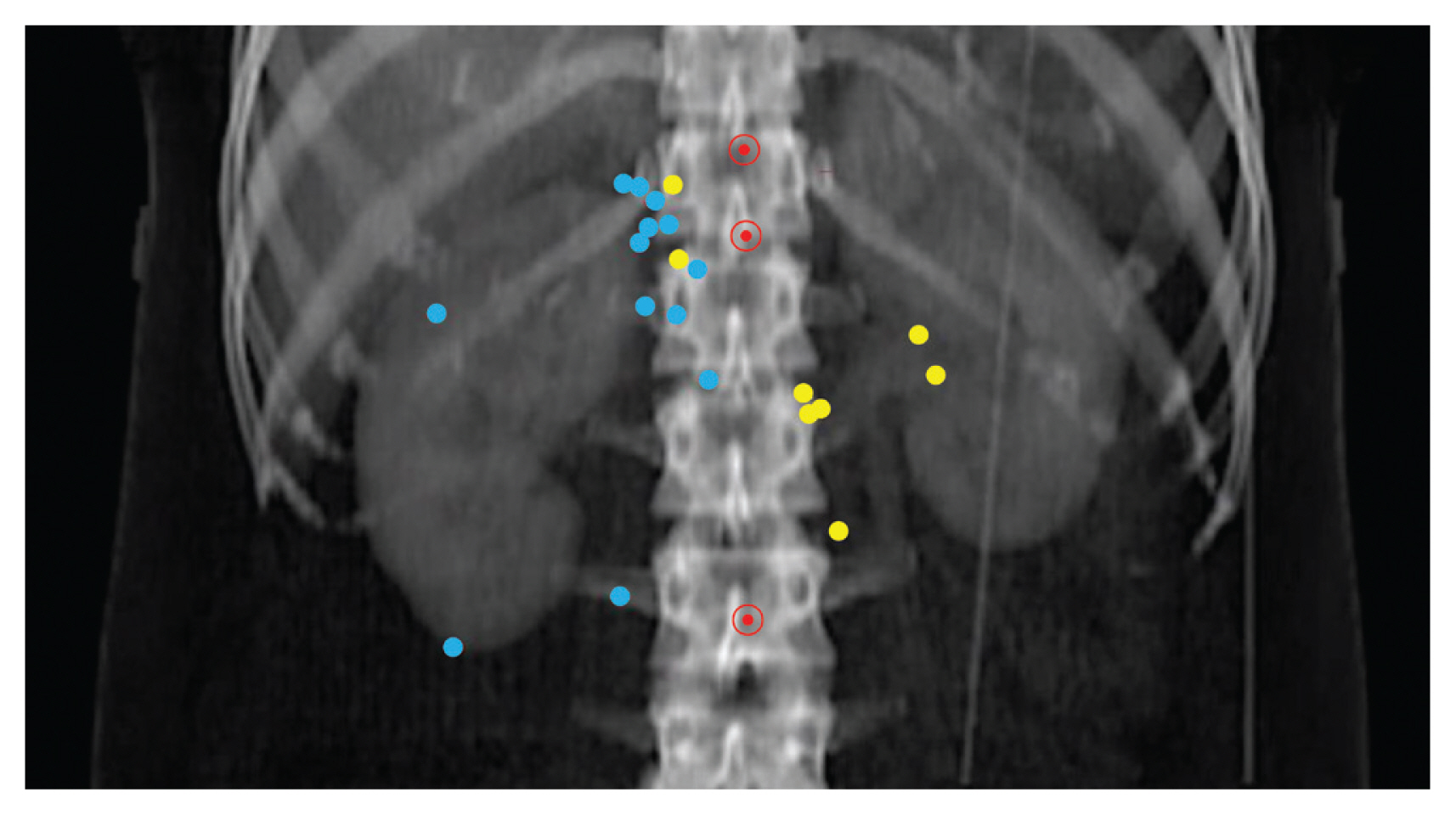
The median follow-up duration was 56 months (range, 1 to 128 months). Tumor extension to renal vessels or the inferior vena cava (IVC) and Fuhrman’s nuclear grade IV were identified as independent risk factors of LRR. The 5-year actuarial LRR rates in groups with no risk factor, one risk factor, and two risk factors were 2.3%, 19.8%, and 30.8%, respectively (p < 0.001). The locations of LRR were distributed as follows: aortocaval area (n=2), paraaortic area (n=4), retrocaval area (n=5), and tumor bed (n=11). No LRR was observed above the celiac axis (CA) or under the inferior mesenteric artery (IMA).
Conclusion
Tumor extension to renal vessels or the IVC and Fuhrman’s nuclear grade IV were the independent risk factors associated with LRR after RN for pT3-4 RCC. The locations of LRR after RN for RCC were distributed in the tumor bed and regional lymphatic area from the bifurcation of the CA to that of the IMA.
Keyword
Figure
Reference
-
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34.
Article2. Lipworth L, Morgans AK, Edwards TL, Barocas DA, Chang SS, Herrell SD, et al. Renal cell cancer histological subtype distribution differs by race and sex. BJU Int. 2016; 117:260–5.
Article3. National Comprehensive Cancer Network. Kidney cancer (version 1.2020) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2020. [cited 2020 Jun 7]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf .4. Kim SP, Weight CJ, Leibovich BC, Thompson RH, Costello BA, Cheville JC, et al. Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology. 2011; 78:1101–6.
Article5. Lenis AT, Donin NM, Johnson DC, Faiena I, Salmasi A, Drakaki A, et al. Adjuvant therapy for high risk localized kidney cancer: emerging evidence and future clinical trials. J Urol. 2018; 199:43–52.
Article6. Jhavar S, Swanson G, Pruszynski J. Risk factors for locoregional relapse after radical nephrectomy. Asia Pac J Clin Oncol. 2018; 14:192–7.
Article7. Escudier B, Porta C, Schmidinger M, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019; 30:706–20.8. Ljungberg B, Albiges L, Bedke K, Bex A, Capitanio U, Giles RH, et al. European Association of Urology guidelines on renal cell carcinoma [Internet]. Arhem: European Association of Urology;2019. [cited 2020 Jun 7]. Available from: https://uroweb.org/guideline/renal-cell-carcinoma/ .9. Ward RD, Tanaka H, Campbell SC, Remer EM. 2017 AUA renal mass and localized renal cancer guidelines: imaging implications. Radiographics. 2018; 38:2021–33.
Article10. Finney R. The value of radiotherapy in the treatment of hypernephroma: a clinical trial. Br J Urol. 1973; 45:258–69.11. Kjaer M, Iversen P, Hvidt V, Bruun E, Skaarup P, Bech Hansen J, et al. A randomized trial of postoperative radiotherapy versus observation in stage II and III renal adenocarcinoma: a study by the Copenhagen Renal Cancer Study Group. Scand J Urol Nephrol. 1987; 21:285–9.12. Campi R, Sessa F, Di Maida F, Greco I, Mari A, Takacova T, et al. Templates of lymph node dissection for renal cell carcinoma: a systematic review of the literature. Front Surg. 2018; 5:76.
Article13. Amin MB, Edge SB, Green FL. AJCC cancer staging manual. 8th ed.New York: Springer;2017.14. Haijun Y, Qiuji W, Zhenming F, Yong H, Zhengkai L, Conghua X, et al. A new approach to delineating lymph node target volumes for post-operative radiotherapy in gastric cancer: a phase II trial. Radiother Oncol. 2015; 116:245–51.
Article15. Chin AI, Lam JS, Figlin RA, Belldegrun AS. Surveillance strategies for renal cell carcinoma patients following nephrectomy. Rev Urol. 2006; 8:1–7.16. Liu G, Ma Y, Wang S, Han X, Gao D. Laparoscopic versus open radical nephrectomy for renal cell carcinoma: a systematic review and meta-analysis. Transl Oncol. 2017; 10:501–10.
Article17. Fayek IS, Habashy HF, Habashy NF. Isolated locoregional recurrence after radical nephrectomy for renal cell carcinoma: a study of 22 patients. J Egypt Natl Canc Inst. 2014; 26:161–6.18. Zisman A, Pantuck AJ, Dorey F, Said JW, Shvarts O, Quintana D, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001; 19:1649–57.
Article19. Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003; 97:1663–71.20. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002; 168:2395–400.
Article21. Karakiewicz PI, Briganti A, Chun FK, Trinh QD, Perrotte P, Ficarra V, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram. J Clin Oncol. 2007; 25:1316–22.
Article22. Capitanio U, Leibovich BC. The rationale and the role of lymph node dissection in renal cell carcinoma. World J Urol. 2017; 35:497–506.
Article23. Blom JH, van Poppel H, Marechal JM, Jacqmin D, Schroder FH, de Prijck L, et al. Radical nephrectomy with and without lymph-node dissection: final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol. 2009; 55:28–34.
Article24. Kates M, Lavery HJ, Brajtbord J, Samadi D, Palese MA. Decreasing rates of lymph node dissection during radical nephrectomy for renal cell carcinoma. Ann Surg Oncol. 2012; 19:2693–9.
Article25. Ulutin HC, Aksu G, Fayda M, Kuzhan O, Tahmaz L, Beyzadeoglu M. The value of postoperative radiotherapy in renal cell carcinoma: a single-institution experience. Tumori. 2006; 92:202–6.
Article26. Safwat A, Shouman T, Hamada E, El-Naggar H, Mohamed NH. Post operative radiotherapy improves disease free but not overall survival in high risk renal cell carcinoma patients. J Egypt Natl Cancer Inst. 2000; 12:17–22.27. Makarewicz R, Zarzycka M, Kulinska G, Windorbska W. The value of postoperative radiotherapy in advanced renal cell cancer. Neoplasma. 1998; 45:380–3.28. Kao GD, Malkowicz SB, Whittington R, D’Amico AV, Wein AJ. Locally advanced renal cell carcinoma: low complication rate and efficacy of postnephrectomy radiation therapy planned with CT. Radiology. 1994; 193:725–30.
Article29. Stein M, Kuten A, Halpern J, Coachman NM, Cohen Y, Robinson E. The value of postoperative irradiation in renal cell cancer. Radiother Oncol. 1992; 24:41–4.
Article30. Tunio MA, Hashmi A, Rafi M. Need for a new trial to evaluate postoperative radiotherapy in renal cell carcinoma: a meta-analysis of randomized controlled trials. Ann Oncol. 2010; 21:1839–45.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Renal Cell Carcinoma with Hyperglycemia Corrected after Radical Nephrectomy
- The Treatment Outcomes of a Partial Nephrectomy in the Management of Renal Cell Carcinomas
- Patterns of Tumor Recurrence after Nephron Sparing Surgery for Renal Cell Carcinoma
- Reconsideration of the Necessity of Routine Ipsilateral Adrenalectomy during Radical Nephrectomy for Renal Cell Carcinoma
- Metastases to Ureteral Stump and Bladder from Renal Cell Carcinoma