Cancer Res Treat.
2022 Jan;54(1):182-198. 10.4143/crt.2020.578.
Vps34 Inhibits Hepatocellular Carcinoma Invasion by Regulating Endosome-Lysosome Trafficking via Rab7-RILP and Rab11
- Affiliations
-
- 1Department of Pathology, School of Basic Medical Sciences, Shanghai Medical College, Fudan University, Shanghai, China
- 2Department of Pathology, Huashan Hospital, Fudan University, Shanghai, China
- 3Department of Pathology, Yantai Yuhuangding Hospital of Qingdao University, Yantai, China
- KMID: 2524599
- DOI: http://doi.org/10.4143/crt.2020.578
Abstract
- Purpose
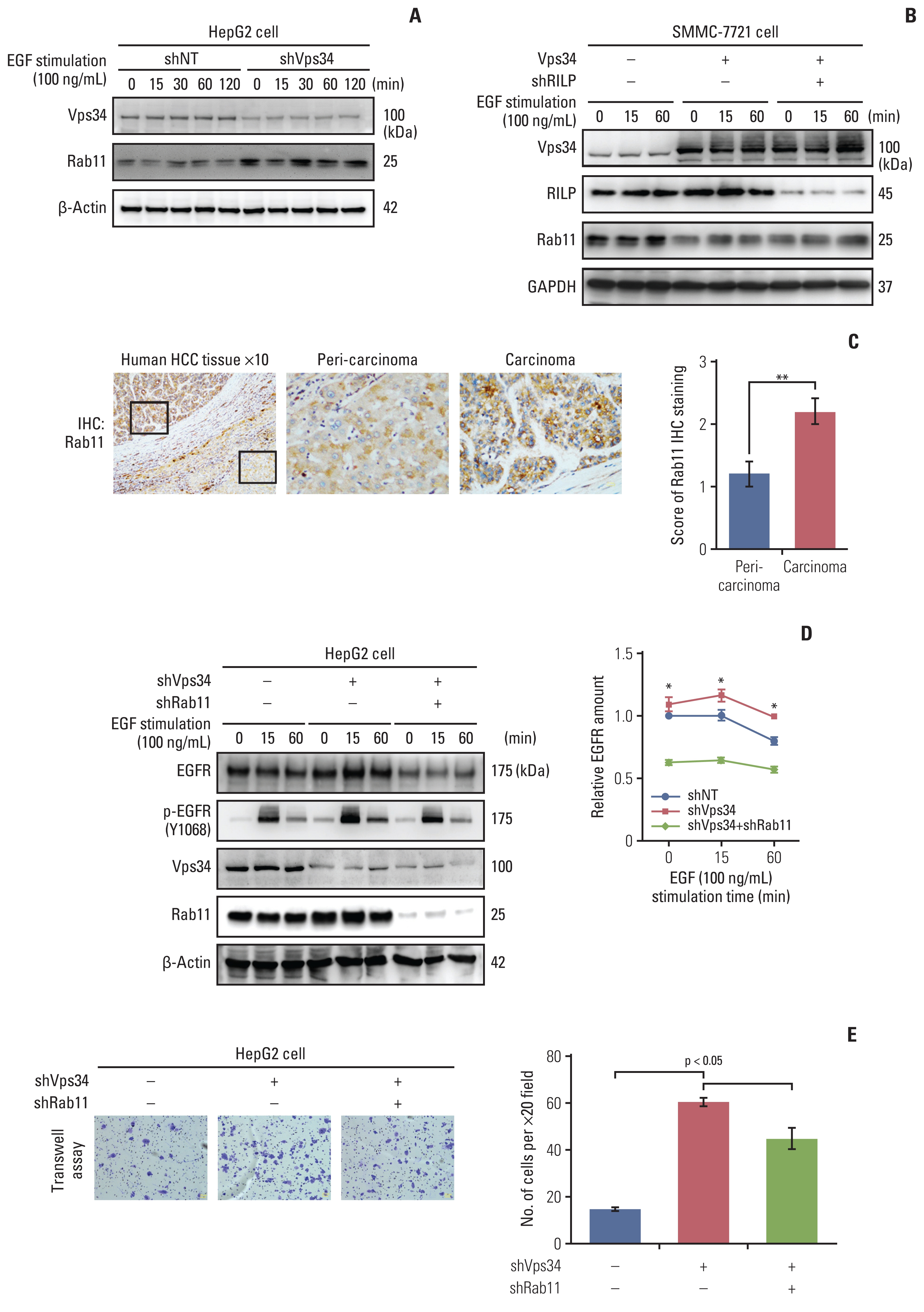
The role of vacuolar protein sorting 34 (Vps34), an indispensable protein required for cell vesicular trafficking, in the biological behavior of hepatocellular carcinoma (HCC) has yet to be studied.
Materials and Methods
In the present study, the expression of Vps34 in HCC and the effect of Vps34 on HCC cell invasion was detected both in vivo and in vitro. Furthermore, by modulating the RILP and Rab11, which regulate juxtanuclear lysosome aggregation and recycling endosome respectively, the underlying mechanism was investigated.
Results
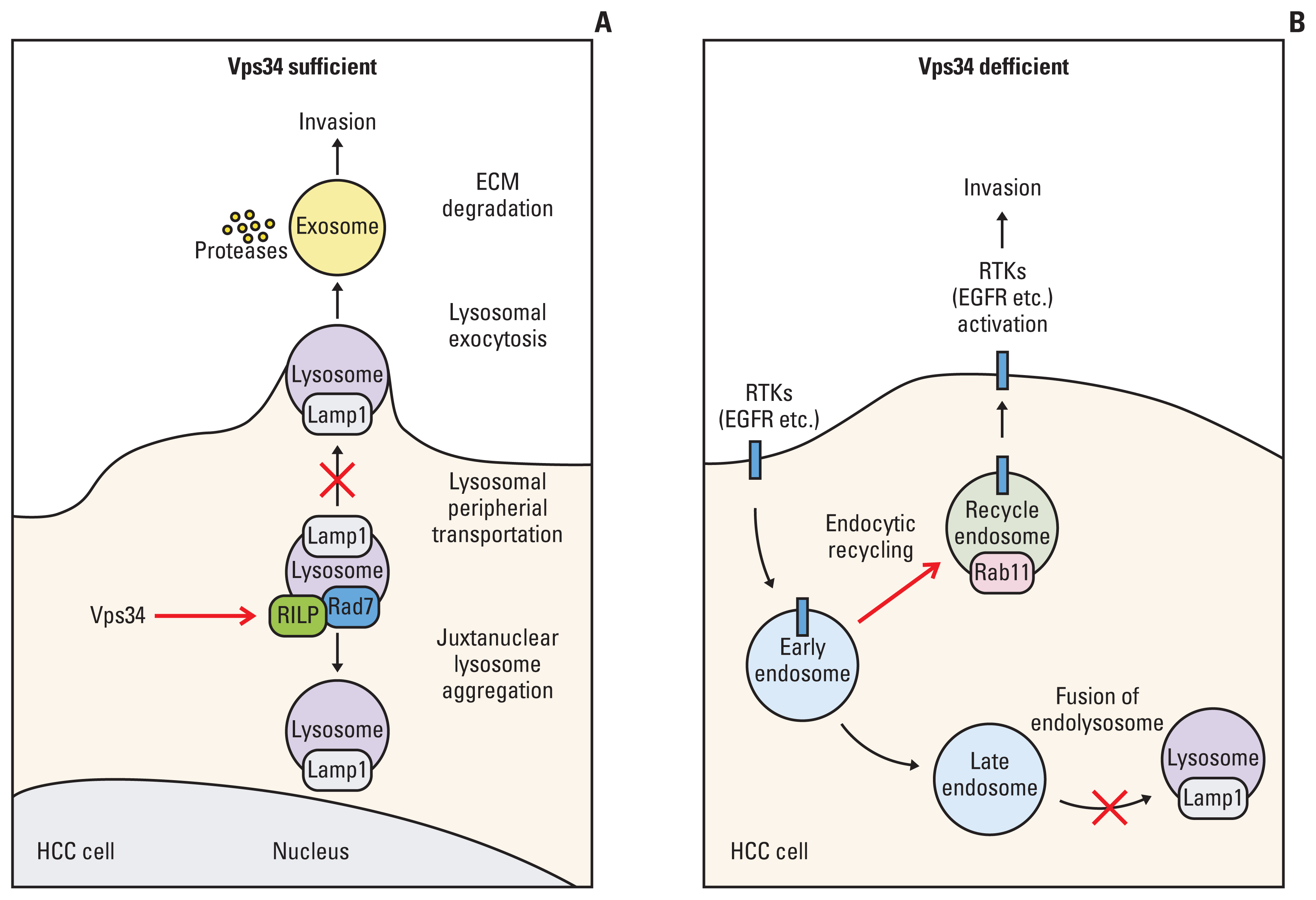
Vps34 was significantly decreased in HCC and negatively correlated with the HCC invasiveness both in vivo and in vitro. Moreover, Vps34 could promote lysosomal juxtanuclear accumulation, reduce the invasive ability of HCC cells via the Rab7-RILP pathway. In addition, the deficiency of Vps34 in HCC cells affected the endosome-lysosome system, resulting in enhanced Rab11 mediated endocytic recycling of cell surface receptor and increased invasion of HCC cells.
Conclusion
Our study reveals that Vps34 acts as an invasion suppressor in HCC cells, and more importantly, the endosome-lysosome trafficking regulated by Vps34 has the potential to become a target pathway in HCC treatment.
Keyword
Figure
Reference
-
References
1. Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci. 2006; 119:605–14.
Article2. Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005; 102:14238–43.
Article3. Lamb CA, Dooley HC, Tooze SA. Endocytosis and autophagy: shared machinery for degradation. Bioessays. 2013; 35:34–45.
Article4. Perera RM, Zoncu R. The lysosome as a regulatory hub. Annu Rev Cell Dev Biol. 2016; 32:223–53.
Article5. Mellman I, Yarden Y. Endocytosis and cancer. Cold Spring Harb Perspect Biol. 2013; 5:a016949.
Article6. Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature. 2010; 465:942–6.
Article7. Wirth M, Joachim J, Tooze SA. Autophagosome formation: the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin Cancer Biol. 2013; 23:301–9.8. Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999; 402:672–6.
Article9. LI M, Xiong J. Interpretation of guidelines for diagnosis and treatment of primary liver cancer (2017 edition). Chin J Gen Surg. 2019; 28:785–9.10. van Zijl F, Krupitza G, Mikulits W. Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res. 2011; 728:23–34.
Article11. Ma Y, Ma L, Guo Q, Zhang S. Expression of bone morphogenetic protein-2 and its receptors in epithelial ovarian cancer and their influence on the prognosis of ovarian cancer patients. J Exp Clin Cancer Res. 2010; 29:85.
Article12. Ye QH, Zhu WW, Zhang JB, Qin Y, Lu M, Lin GL, et al. GOLM1 modulates EGFR/RTK cell-surface recycling to drive hepatocellular carcinoma metastasis. Cancer Cell. 2016; 30:444–58.
Article13. Yan Y, Backer JM. Regulation of class III (Vps34) PI3Ks. Biochem Soc Trans. 2007; 35:239–41.
Article14. Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011; 91:119–49.
Article15. Al-Akhrass H, Naves T, Vincent F, Magnaudeix A, Durand K, Bertin F, et al. Sortilin limits EGFR signaling by promoting its internalization in lung cancer. Nat Commun. 2017; 8:1182.
Article16. Bechtel W, Helmstadter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, et al. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol. 2013; 24:727–43.
Article17. Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A. 2012; 109:2003–8.
Article18. Zhou X, Wang L, Hasegawa H, Amin P, Han BX, Kaneko S, et al. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc Natl Acad Sci U S A. 2010; 107:9424–9.
Article19. Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011; 13:453–60.
Article20. Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009; 185:11–9.
Article21. Jordens I, Fernandez-Borja M, Marsman M, Dusseljee S, Janssen L, Calafat J, et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol. 2001; 11:1680–5.
Article22. Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex: at the crossroads of autophagy and beyond. Trends Cell Biol. 2010; 20:355–62.23. Itakura E, Mizushima N. Atg14 and UVRAG: mutually exclusive subunits of mammalian Beclin 1-PI3K complexes. Autophagy. 2009; 5:534–6.
Article24. Muller PA, Trinidad AG, Timpson P, Morton JP, Zanivan S, van den Berghe PV, et al. Mutant p53 enhances MET trafficking and signalling to drive cell scattering and invasion. Oncogene. 2013; 32:1252–65.
Article25. Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, et al. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009; 139:1327–41.
Article26. Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996; 135:913–24.
Article27. Dong Q, Fu L, Zhao Y, Du Y, Li Q, Qiu X, et al. Rab11a promotes proliferation and invasion through regulation of YAP in non-small cell lung cancer. Oncotarget. 2017; 8:27800–11.
Article28. Gong X, Liu J, Zhang X, Dong F, Liu Y, Wang P. Rab11 Functions as an oncoprotein via nuclear factor kappa B (NF-kappaB) signaling pathway in human bladder carcinoma. Med Sci Monit. 2018; 24:5093–101.29. Zhu W, Li MC, Wang FR, Mackenzie GG, Oteiza PI. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem Pharmacol. 2020; 175:113923.
Article30. Sheng W, Shi X, Lin Y, Tang J, Jia C, Cao R, et al. Musashi2 promotes EGF-induced EMT in pancreatic cancer via ZEB1-ERK/MAPK signaling. J Exp Clin Cancer Res. 2020; 39:16.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulatory Mechanisms Governing the Autophagy-Initiating VPS34 Complex and Its inhibitors
- Hepatitis B virus X Protein Promotes Liver Cancer Progression through Autophagy Induction in Response to TLR4 Stimulation
- Hepatitis B virus X Protein Promotes Liver Cancer Progression through Autophagy Induction in Response to TLR4 Stimulation
- Bile Duct Invasion of Hepatocellular Carcinoma
- Inferior Vena Cava Invasion of Hepatocellular Carcinoma