J Korean Neurosurg Soc.
2022 Jan;65(1):138-144. 10.3340/jkns.2020.0360.
Type 1.5 Split Cord Malformation : A New Theory of Pathogenesis
- Affiliations
-
- 1Department of Neurosurgery, the First Medical Center of Chinese PLA General Hospital, Beijing, China
- 2Medical School, Nankai University, Tianjin, China
- 3Department of Neurosurgery, Yuquan Hospital, Beijing, China
- KMID: 2523922
- DOI: http://doi.org/10.3340/jkns.2020.0360
Abstract
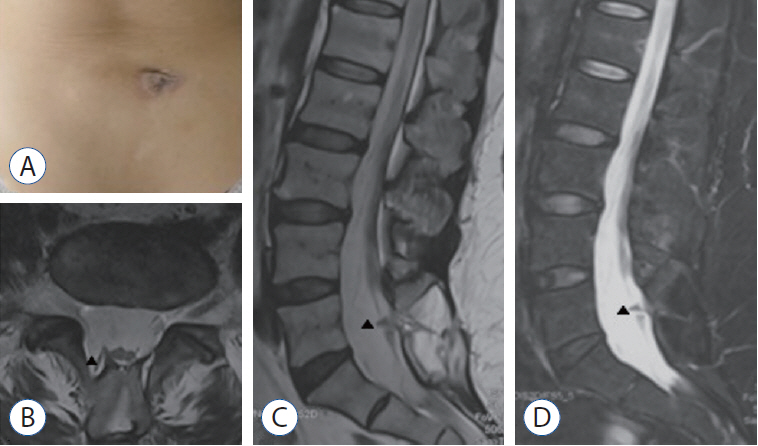
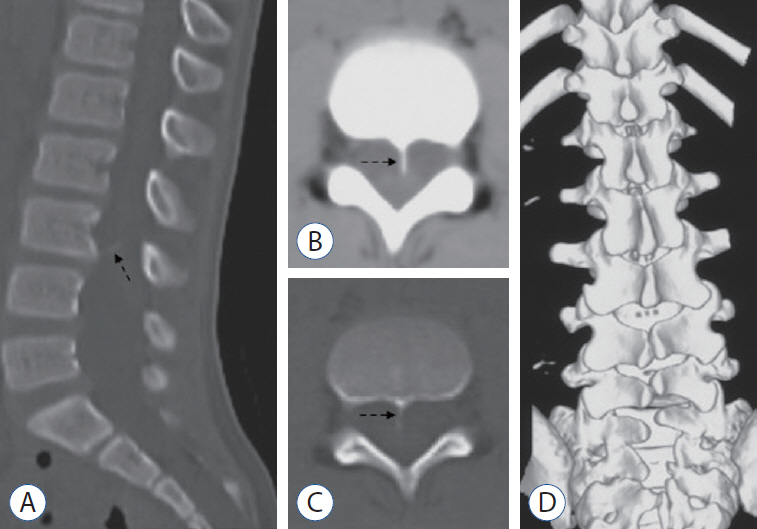
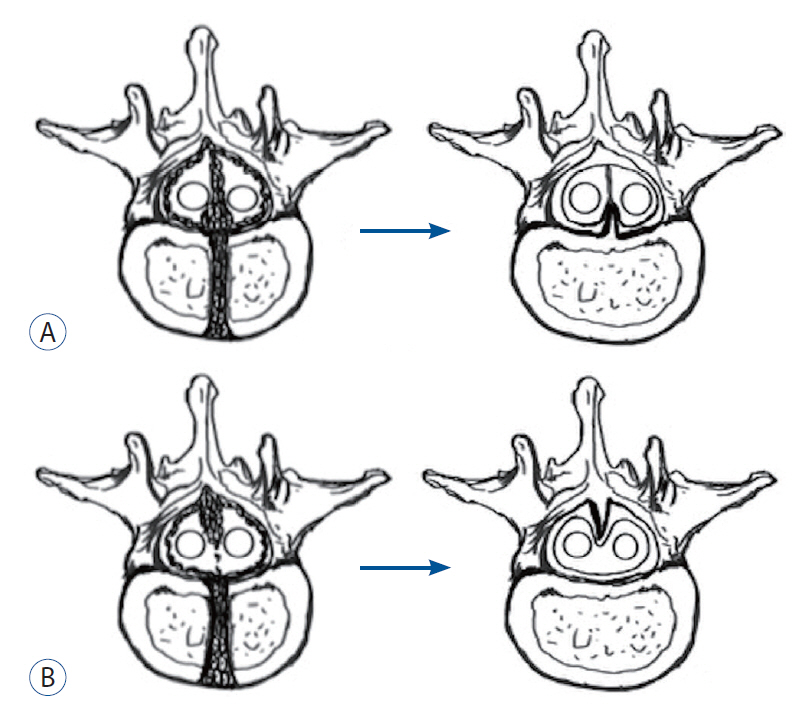
- To report two cases of type 1.5 split cord malformation (SCM), a subtype of SCM with combined characteristics of types I and II and to review the relevant literature and propose a new possible pathogenetic theory for type 1.5 SCM. A 52-year-old woman had hemicords within a single dural sac with a dorsal bony septum at the L5 level. A 9-year-old boy had hemicords within a single dural sac with a ventral bony septum and fibrous extension at the L3 level. Both patients underwent microsurgical treatments for removing the bony septum, detethering the spinal cord, and sectioning the filum terminale. The surgical procedure revealed an extradural partial bony septum and hemicords within an intact single dural sac in each patient. Both patients were discharged from the hospital without de novo nerve dysfunction. Published cases have validated that types I and II SCM can overlap. We recommend recent type 1.5 SCM as a normative terminology for this overlapping SCM and report two rare cases of this SCM. We propose an associated pathogenesis consisting of uneven distribution and regression to explain type 1.5 SCM. Furthermore, we postulate that the amount of condensing meninx primitiva might determine whether the left bony septum has fibrous extensions to the opposite dura in type 1.5 SCM.
Keyword
Figure
Reference
-
References
1. Akay KM, Izci Y, Baysefer A. Dorsal bony septum: a split cord malformation variant. Pediatr Neurosurg. 36:225–228. 2002.
Article2. Akay KM, Izci Y, Baysefer A, Timurkaynak E. Composite type of split cord malformation: two different types at three different levels: case report. J Neurosurg. 102(4 Suppl):436–438. 2005.
Article3. Basak M, Ozel A, Erturk M. An unusual case of diastematomyelia. Presence of one dural sheath associated with a bony spur. Acta Radiol. 43:626. 2002.
Article4. Bruce A, M’Donald S, Pirie JHH. A second case of partial doubling of the spinal cord. Rev Neurol Psychiatr. 4:6. 1906.5. Chandra PS, Kamal R, Mahapatra AK. An unusual case of dorsally situated bony spur in a lumbar split cord malformation. Pediatr Neurosurg. 31:49–52. 1999.
Article6. Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 221:117–145. 2001.
Article7. Erşahin Y. An unusual split cord malformation. Pediatr Neurosurg. 32:109. 2000.
Article8. Garg K, Mahapatra AK, Tandon V. A rare case of type 1 C split cord malformation with single dural sheath. Asian J Neurosurg. 10:226–228. 2015.
Article9. Herren RY, Edwards JE. Diplomyelia (duplication of the spinal cord). Arch Pathol. 30:1203–1213. 1940.10. Hertwig O. Urmund und spina bifida. Archiv f mikrosk Anatomie. 39:353–502. 1892.
Article11. Izci Y, Kural C. Composite type of split cord malformation: rare and difficult to explain. Pediatr Neurosurg. 47:461. 2011.
Article12. Lichtenstein BW. Spinal dysraphism: spina bifida and myelodysplasia. Arch Neur Psych. 44:792–810. 1940.13. Marr GE, Uihlein A. Diplomyelia and compression of the spinal cord and not of the cauda equina, by a congenital anomaly of the third lumbar vertebra. Surg Clin North Am. 24:963–977. 1944.14. Maxwell HP, Bucy PC. Diastematomyelia; report of a clinical case. J Neuropathol Exp Neurol. 5:165–167. 1946.
Article15. Meena RK, Doddamani RS, Gurjar HK, Kumar A, Chandra PS. Type 1.5 split cord malformations: an uncommon entity. World Neurosurg. 133:142–149. 2020.
Article16. Pang D, Dias MS, Ahab-Barmada M. Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 31:451–480. 1992.17. Pickles W. Duplication of the spinal cord; an account of a clinical example with a consideration of other reports. J Neurosurg. 6:324–331. 1949.18. Salunke P, Kovai P, Malik V, Sharma M. Mixed split cord malformation: are we missing something? Clin Neurol Neurosurg. 113:774–778. 2011.
Article19. Singh PK, Khandelwal A, Singh A, Ailawadhi P, Gupta D, Mahapatra AK. Long-segment type 1 split cord malformation with two-level split cord malformation and a single dural sac at the lower split. Pediatr Neurosurg. 47:227–229. 2011.
Article20. Vaishya S, Kumarjain P. Split cord malformation: three unusual cases of composite split cord malformation. Childs Nerv Syst. 17:528–530. 2001.
Article21. Van Aalst J, Beuls EA, Vles JS, Cornips EM, van Straaten HW. The intermediate type split cord malformation: hypothesis and case report. Childs Nerv Syst. 21:1020–1024. 2005.
Article22. Xu X, Li C, Takahashi K, Slavkin HC, Shum L, Deng CX. Murine fibroblast growth factor receptor 1alpha isoforms mediate node regression and are essential for posterior mesoderm development. Dev Biol. 208:293–306. 1999.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sacrococcygeal Teratoma with Split Spinal Cord Malformation
- Split Cord Malformation Combined with Tethered Cord Syndrome in an Adult
- Prenatally diagnosed split hand malformation at 16 weeks' gestation by 3D ultrasonography
- We Are Cautious to Use the Term, ‘Split Cord Malformation Type 1.5’
- Anesthetic management of a patient with Arnold-Chiari malformation type I with associated syringomyelia: A case report