Ann Surg Treat Res.
2022 Jan;102(1):1-9. 10.4174/astr.2022.102.1.1.
Hepatectomy outcomes in patients with hepatitis C virusrelated hepatocellular carcinoma with or without cirrhosis
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Pathology and Translational Genomics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Surgery, Digestive Disease and Surgery Institute, Cleveland Clinic, Cleveland, OH, USA
- KMID: 2523764
- DOI: http://doi.org/10.4174/astr.2022.102.1.1
Abstract
- Purpose
Hepatocellular carcinoma (HCC) is rare in HCV patients without cirrhosis, and little is known about the postoperative results of these patients. The present study compares the outcomes of cirrhotic and non-cirrhotic groups after liver resection (LR) in solitary HCV-related HCC patients and identifies risk factors for prognosis according to the presence or absence of cirrhosis in these patients.
Methods
Two hundred and 7 adult hepatectomy patients with treatment-naïve solitary HCV-related HCC were identified prospectively at our institution between July 2005 and May 2019.
Results
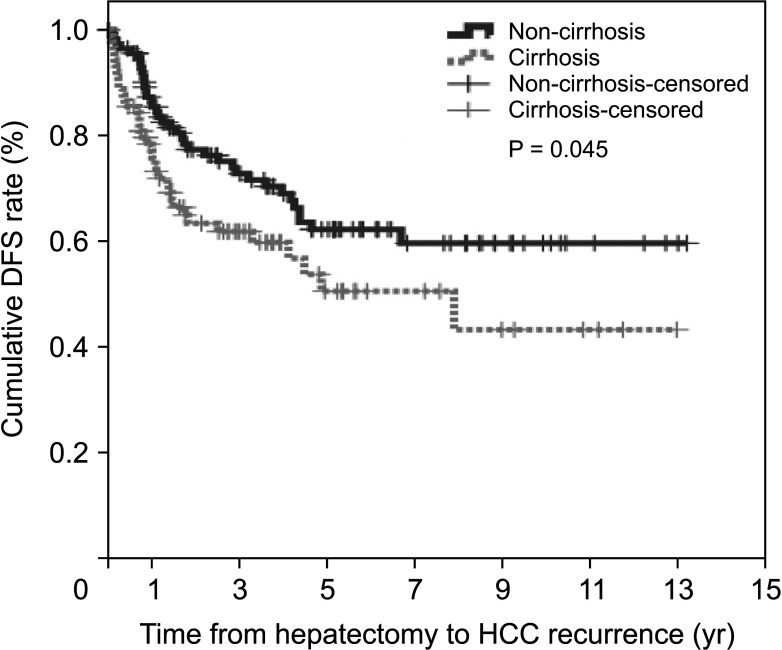
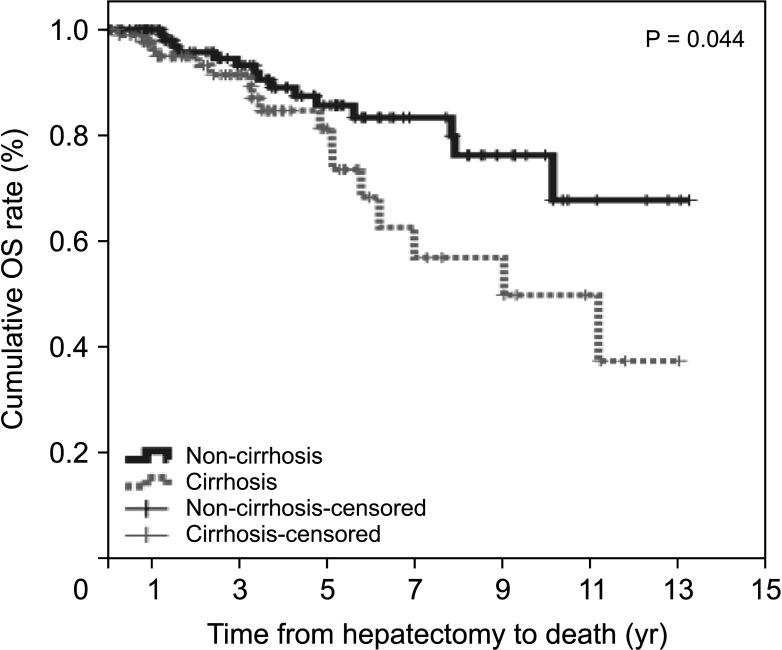
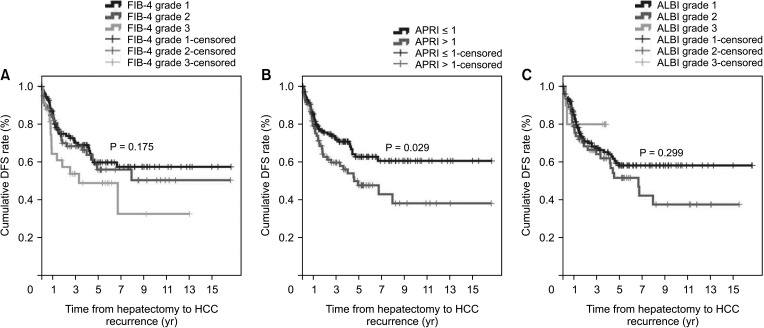
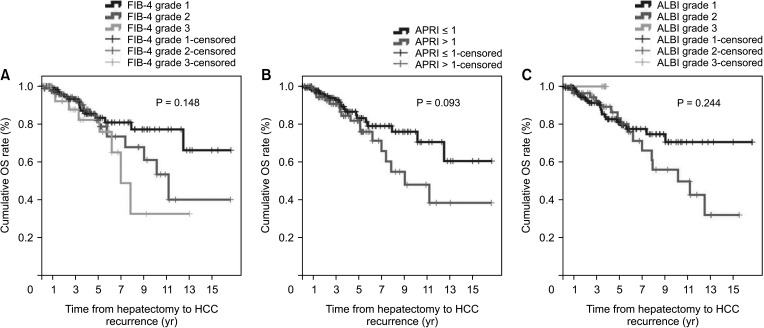
The non-cirrhotic group had better liver function than the cirrhotic group based on platelet count, liver function tests, liver stiffness measurement, and indocyanine green retention rate at 15 minutes but were older than the cirrhotic group. Consistently, noninvasive markers in the cirrhotic group were significantly higher than in the non-cirrhotic group. The cumulative disease-free survival and overall survival in the non-cirrhotic group were significantly higher than in the cirrhotic group. HCC recurrence was related to major LR and α-FP of >40 ng/mL and death was related to long hospitalization and α-FP of >40 ng/mL in multivariate analysis. Noninvasive markers and the presence of cirrhosis were not related to HCC recurrence or death in multivariate analyses.
Conclusion
The cirrhotic group showed poor prognosis due to poor liver function after LR compared to the non-cirrhotic group, but this was not sustained in multivariate analysis. The factors influencing HCC recurrence and death were different in the cirrhotic and non-cirrhotic groups.
Keyword
Figure
Cited by 1 articles
-
A systematic review and meta-analysis of blood transfusion rates during liver resection by country
Seonju Kim, Yun Kyung Jung, Kyeong Geun Lee, Kyeong Sik Kim, Hanjun Kim, Dongho Choi, Sumi Lee, Boyoung Park
Ann Surg Treat Res. 2023;105(6):404-416. doi: 10.4174/astr.2023.105.6.404.
Reference
-
1. Park SH, Plank LD, Suk KT, Park YE, Lee J, Choi JH, et al. Trends in the prevalence of chronic liver disease in the Korean adult population, 1998-2017. Clin Mol Hepatol. 2020; 26:209–215. PMID: 31679316.
Article2. Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014; 61(1 Suppl):S58–S68. PMID: 25443346.
Article3. Goodgame B, Shaheen NJ, Galanko J, El-Serag HB. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: publication bias? Am J Gastroenterol. 2003; 98:2535–2542. PMID: 14638360.
Article4. Alotaibi AS, Alghamdi W, Marotta P, Qumosani K. A266 Hepatocellular carcinoma prevalence in non-cirrhotic hepat it is C pat ients. J Can Assoc Gastroenterol. 2018; 1(Suppl 2):383–384.5. Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010; 42:341–347. PMID: 19828388.
Article6. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Differences between hepatocellular carcinoma and hepatitis B virus infection in patients with and without cirrhosis. Ann Surg Oncol. 2014; 21:458–465. PMID: 24132624.
Article7. Korean Liver Cancer Association. National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver. 2019; 13:227–299. PMID: 31060120.8. Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999; 229:210–215. PMID: 10024102.9. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003; 38:518–526. PMID: 12883497.
Article10. Zhang Y, Wang R, Yang X. FIB-4 index serves as a noninvasive prognostic biomarker in patients with hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore). 2018; 97:e13696. PMID: 30572498.11. Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol. 2012; 12:14. PMID: 22333407.
Article12. Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016; 103:725–734. PMID: 27005482.
Article13. Zou H, Wen Y, Yuan K, Miao XY, Xiong L, Liu KJ. Combining albumin-bilirubin score with future liver remnant predicts post-hepatectomy liver failure in HBV-associated HCC patients. Liver Int. 2018; 38:494–502. PMID: 28685924.
Article14. Cheng J, Zhao P, Liu J, Liu X, Wu X. Preoperative aspartate aminotransferase-to-platelet ratio index (APRI) is a predictor on postoperative outcomes of hepatocellular carcinoma. Medicine (Baltimore). 2016; 95:e5486. PMID: 27902606.
Article15. Toyoda H, Kumada T, Tada T, Kaneoka Y, Maeda A. A laboratory marker, FIB-4 index, as a predictor for long-term outcomes of hepatocellular carcinoma patients after curative hepatic resection. Surgery. 2015; 157:699–707. PMID: 25704421.16. Hung HH, Su CW, Lai CR, Chau GY, Chan CC, Huang YH, et al. Fibrosis and AST to platelet ratio index predict post-operative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010; 4:691–699. PMID: 21286339.
Article17. Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016; 64:773–780. PMID: 26626497.
Article18. Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Outcomes after curative hepatectomy in patients with non-B non-C hepatocellular carcinoma and hepatitis B virus hepatocellular carcinoma from non-cirrhotic liver. J Surg Oncol. 2014; 110:976–981. PMID: 25171344.
Article19. Sinn DH, Lee HW, Paik YH, Kim DY, Kim YJ, Kim KM, et al. Patterns and outcomes in hepatocellular carcinoma patients with portal vein invasion: a multicenter prospective cohort study. Dig Dis Sci. 2021; 66:315–324. PMID: 32056090.
Article20. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018; 69:154–181. PMID: 29628280.21. Oh IS, Sinn DH, Kang TW, Lee MW, Kang W, Gwak GY, et al. Liver function assessment using albumin-bilirubin grade for patients with very early-stage hepatocellular carcinoma treated with radiofrequency ablation. Dig Dis Sci. 2017; 62:3235–3242. PMID: 28983724.
Article22. Kim JM, Kwon CH, Yoo H, Kim KS, Lee J, Kim K, et al. Which approach is preferred in left hepatocellular carcinoma?: laparoscopic versus open hepatectomy using propensity score matching. BMC Cancer. 2018; 18:668. PMID: 29921239.
Article23. Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J Hepatol. 2019; 11:1–18. PMID: 30705715.
Article24. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43:1317–1325. PMID: 16729309.
Article25. Ahn KS, Kang KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: radiofrequency ablation vs. resection vs. transplantation? Clin Mol Hepatol. 2019; 25:354–359. PMID: 31006225.
Article26. Carrat F, Fontaine H, Dorival C, Simony M, Diallo A, Hezode C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019; 393:1453–1464. PMID: 30765123.
Article27. Bennett S, Baker LK, Martel G, Shorr R, Pawlik TM, Tinmouth A, et al. The impact of perioperative red blood cell transfusions in patients undergoing liver resection: a systematic review. HPB (Oxford). 2017; 19:321–330. PMID: 28161216.
Article28. Hallet J, Mahar AL, Nathens AB, Tsang ME, Beyfuss KA, Lin Y, et al. The impact of perioperative blood transfusions on short-term outcomes following hepatectomy. Hepatobiliary Surg Nutr. 2018; 7:1–10. PMID: 29531938.
Article29. Xun Y, Tian H, Hu L, Yan P, Yang K, Guo T. The impact of perioperative allogeneic blood transfusion on prognosis of hepatocellular carcinoma after radical hepatectomy: a systematic review and meta-analysis of cohort studies. Medicine (Baltimore). 2018; 97:e12911. PMID: 30412094.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Associating microwave ablation and portal vein ligation for staged hepatectomy for the treatment of huge hepatocellular carcinoma with cirrhosis
- Survival rate of hepatectomy according to liver cirrhosis in hepatoculluar carcinoma
- Hepatitis C virus antibodies in patients with hepatocellular carcinoma and liver cirrhosis
- Who Needs Screening for the Early Detection of Hepatocellular Carcinoma ?
- Prognostic Factors and Clinicopathologic Features after Resection of Small Hepatocellular Carcinoma (< or =2 cm)