Endocrinol Metab.
2021 Dec;36(6):1232-1242. 10.3803/EnM.2021.1087.
The Leg Fat to Total Fat Ratio Is Associated with Lower Risks of Non-Alcoholic Fatty Liver Disease and Less Severe Hepatic Fibrosis: Results from Nationwide Surveys (KNHANES 2008–2011)
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 3Institute of Endocrine Research, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2523492
- DOI: http://doi.org/10.3803/EnM.2021.1087
Abstract
- Background
The prevalence of non-alcoholic fatty liver disease (NAFLD) has rapidly increased worldwide. The aim of this study was to investigate whether there is an independent relationship between regional fat distribution, especially leg fat mass, and the presence of NAFLD using nationally representative data in Korea.
Methods
This cross-sectional study analyzed data from 14,502 participants in the Korea National Health and Nutrition Examination Survey 2008 to 2011. Total fat mass, leg fat mass, and appendicular skeletal muscle mass were measured by dual-energy X-ray absorptiometry. Validated NAFLD prediction models and scoring systems for hepatic fibrosis were used.
Results
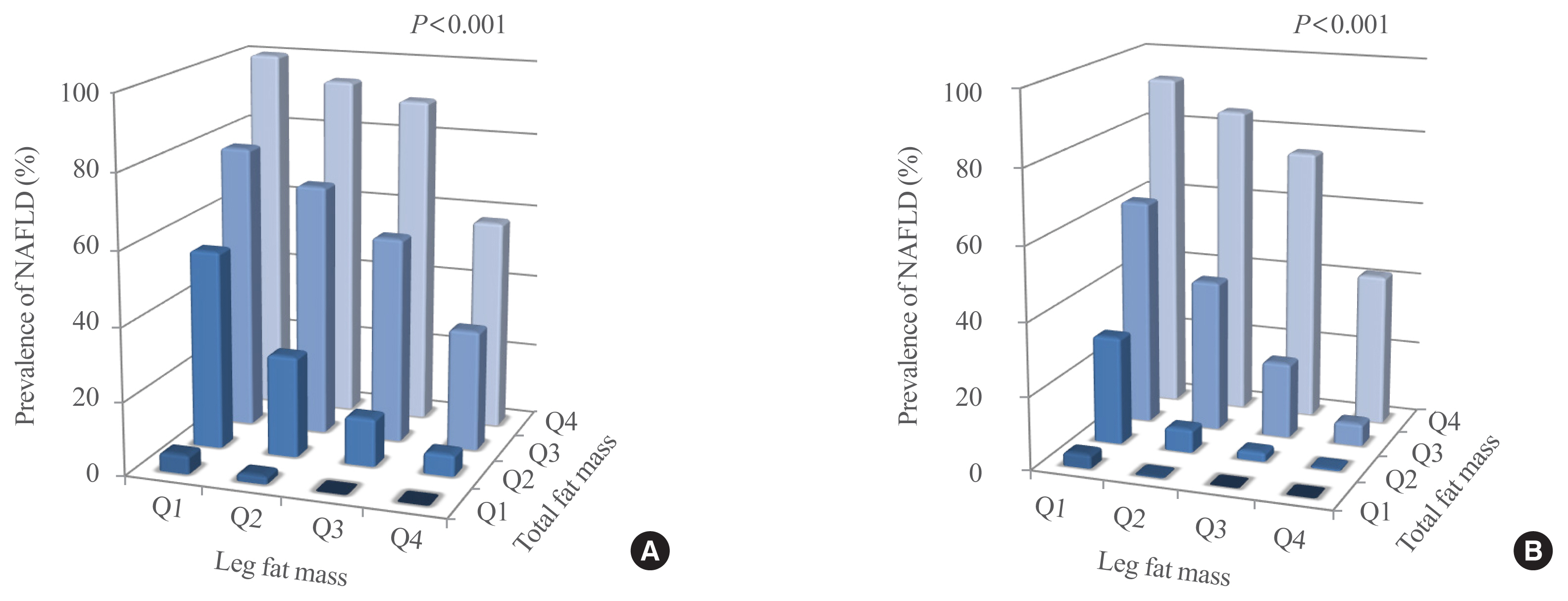
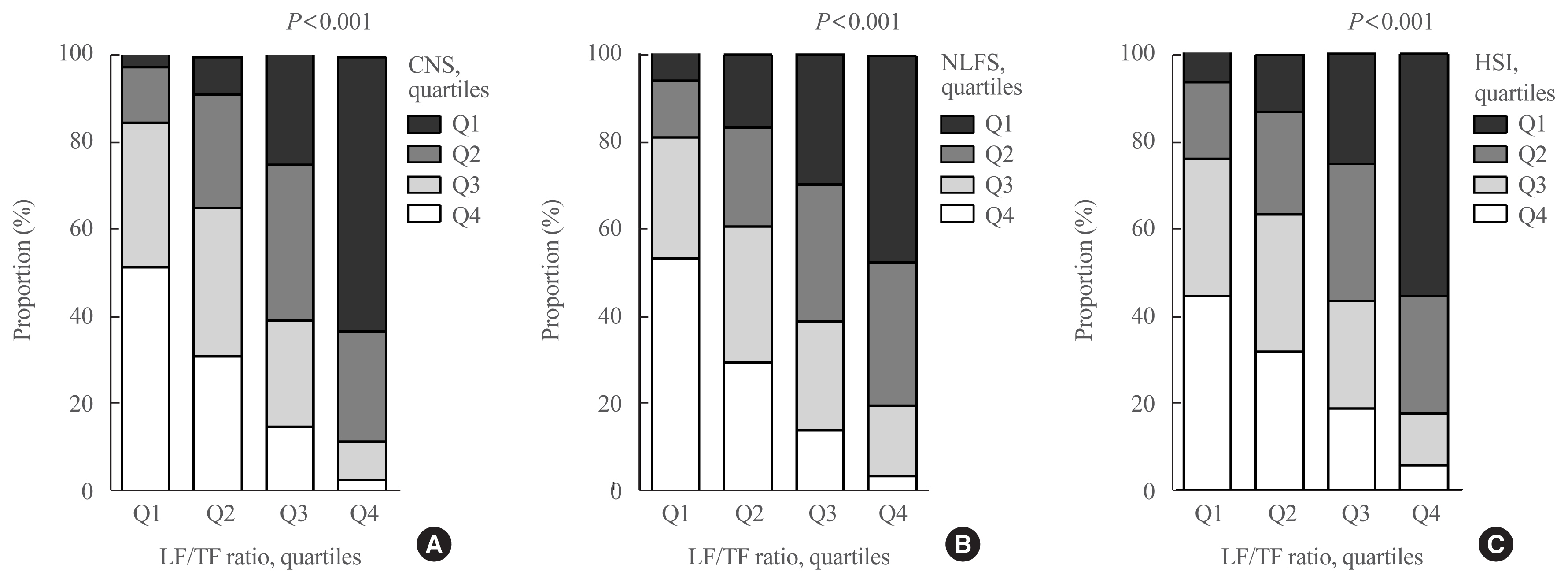
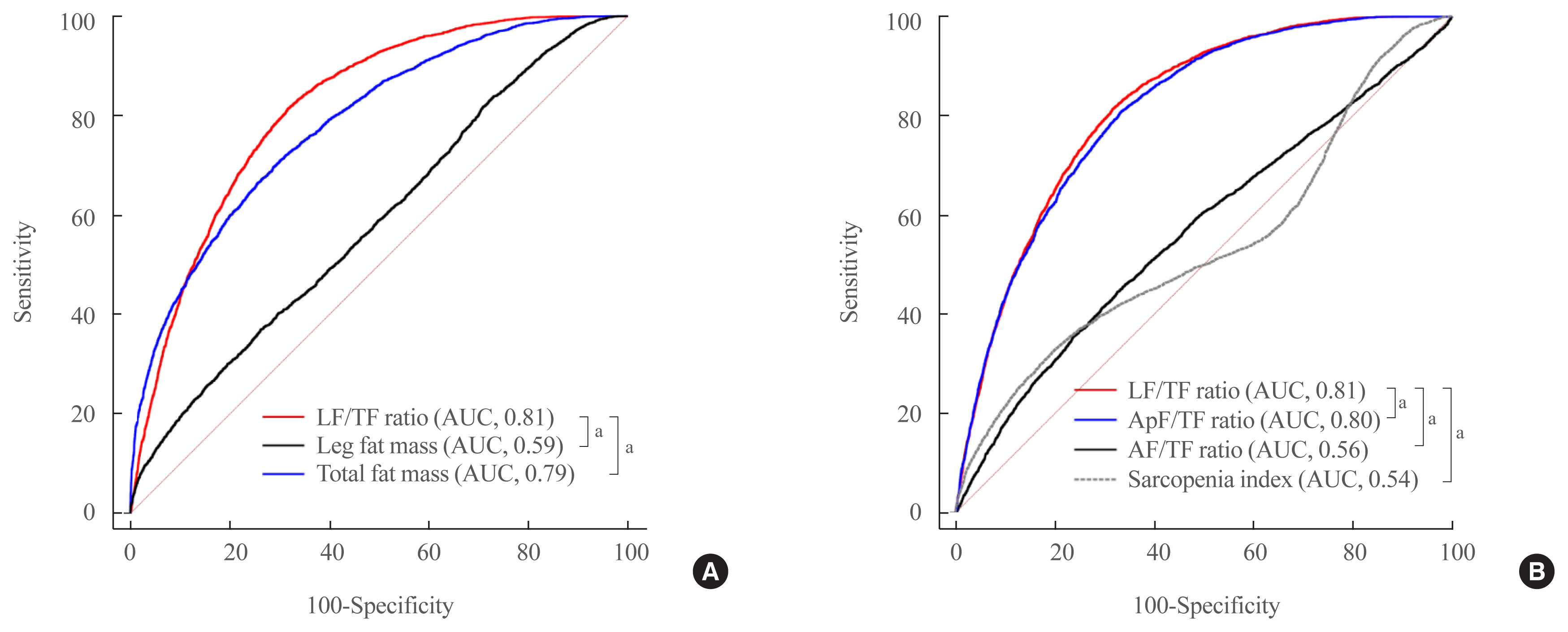
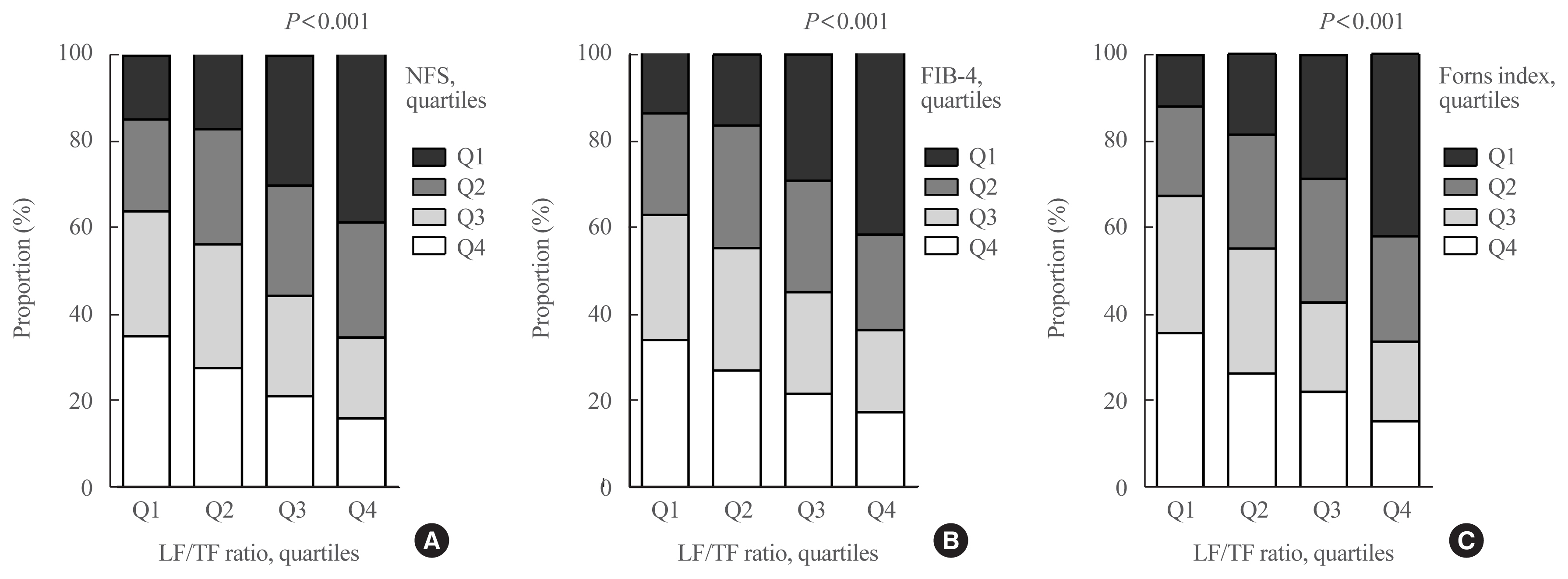
The leg fat to total fat (LF/TF) ratio showed a negative relationship with many factors, including body mass index, waist circumference, blood pressure, fasting blood glucose, and liver enzyme levels. When the LF/TF ratio and indices of hepatic steatosis were stratified by quartiles, the LF/TF ratio showed a negative correlation with the scoring systems that were used. The LF/TF ratio showed better accuracy in predicting NAFLD than total fat mass or leg fat mass alone. After adjusting for various traditional and lifestyle factors, a low LF/TF ratio remained a risk factor for NAFLD. Among NAFLD subjects, the LF/TF ratio showed a negative relationship with hepatic fibrosis.
Conclusion
A lower LF/TF ratio was markedly associated with a higher risk of hepatic steatosis and advanced hepatic fibrosis using various predictive models in a Korean population. Therefore, the LF/TF ratio could be a useful anthropometric parameter to predict NAFLD or advanced hepatic fibrosis.
Figure
Cited by 1 articles
-
Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
Inha Jung, Dae-Jeong Koo, Won-Young Lee
Diabetes Metab J. 2024;48(3):327-339. doi: 10.4093/dmj.2023.0350.
Reference
-
1. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015; 313:2263–73.2. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013; 10:686–90.
Article3. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011; 34:274–85.
Article4. Rhee EJ. Nonalcoholic fatty liver disease and diabetes: an epidemiological perspective. Endocrinol Metab (Seoul). 2019; 34:226–33.
Article5. Han E, Lee YH. Non-alcoholic fatty liver disease: the emerging burden in cardiometabolic and renal diseases. Diabetes Metab J. 2017; 41:430–7.
Article6. Han JM, Kim HI, Lee YJ, Lee JW, Kim KM, Bae JC. Differing associations between fatty liver and dyslipidemia according to the degree of hepatic steatosis in Korea. J Lipid Atheroscler. 2019; 8:258–66.
Article7. Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev. 2016; 17:510–9.
Article8. Choi SI, Chung D, Lim JS, Lee MY, Shin JY, Chung CH, et al. Relationship between regional body fat distribution and diabetes mellitus: 2008 to 2010 Korean National Health and Nutrition Examination Surveys. Diabetes Metab J. 2017; 41:51–9.
Article9. Goodpaster BH, Krishnaswami S, Harris TB, Katsiaras A, Kritchevsky SB, Simonsick EM, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005; 165:777–83.
Article10. Subramanian V, Johnston RD, Kaye P, Aithal GP. Regional anthropometric measures associated with the severity of liver injury in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013; 37:455–63.
Article11. Suzuki A, Abdelmalek MF, Unalp-Arida A, Yates K, Sanyal A, Guy C, et al. Regional anthropometric measures and hepatic fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2010; 8:1062–9.
Article12. Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005; 128:1898–906.
Article13. Lee DH. Noninvasive evaluation of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul). 2020; 35:243–59.
Article14. Lee YH, Bang H, Park YM, Bae JC, Lee BW, Kang ES, et al. Non-laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: development, validation and comparison with other scores. PLoS One. 2014; 9:e107584.
Article15. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008; 57:1441–7.
Article16. Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014; 5:211–8.
Article17. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013; 57:1357–65.
Article18. Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012; 12:2.
Article19. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013; 145:782–9.
Article20. Lee YH, Jung KS, Kim SU, Yoon HJ, Yun YJ, Lee BW, et al. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: nationwide surveys (KNHANES 2008–2011). J Hepatol. 2015; 63:486–93.
Article21. Lee YH, Kim SU, Song K, Park JY, Kim DY, Ahn SH, et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008–2011). Hepatology. 2016; 63:776–86.
Article22. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014; 69:547–58.
Article23. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33(Suppl 1):S62–9.24. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005; 112:2735–52.25. Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J. 2011; 35:561–6.
Article26. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–12.
Article27. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–9.
Article28. Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009; 137:865–72.
Article29. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010; 42:503–8.
Article30. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007; 45:846–54.
Article31. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007; 46:32–6.
Article32. Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002; 36(4 Pt 1):986–92.
Article33. Hu G, Bouchard C, Bray GA, Greenway FL, Johnson WD, Newton RL Jr, et al. Trunk versus extremity adiposity and cardiometabolic risk factors in white and African American adults. Diabetes Care. 2011; 34:1415–8.
Article34. Hsing JC, Nguyen MH, Yang B, Min Y, Han SS, Pung E, et al. Associations between body fat, muscle mass, and nonalcoholic fatty liver disease: a population-based study. Hepatol Commun. 2019; 3:1061–72.
Article35. Alferink LJ, Trajanoska K, Erler NS, Schoufour JD, de Knegt RJ, Ikram MA, et al. Nonalcoholic fatty liver disease in the rotterdam study: about muscle mass, sarcopenia, fat mass, and fat distribution. J Bone Miner Res. 2019; 34:1254–63.
Article36. Wahrenberg H, Lonnqvist F, Arner P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J Clin Invest. 1989; 84:458–67.
Article37. Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007; 56:1369–75.38. Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev. 2002; 18(Suppl 2):S5–9.
Article39. Bolinder J, Engfeldt P, Ostman J, Arner P. Site differences in insulin receptor binding and insulin action in subcutaneous fat of obese females. J Clin Endocrinol Metab. 1983; 57:455–61.
Article40. van Harmelen V, Dicker A, Ryden M, Hauner H, Lonnqvist F, Naslund E, et al. Increased lipolysis and decreased leptin production by human omental as compared with subcutaneous preadipocytes. Diabetes. 2002; 51:2029–36.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ultrastructural changes of hepatic stellate cells in the space of Disse in alcoholic fatty liver
- Non-invasive imaging biomarkers for liver steatosis in non-alcoholic fatty liver disease: present and future
- Clinicopathologic Study of Pulmonary Fat Embolism in Fatty Liver
- Nonalcoholic Fatty Liver Disease and Diabetes: An Epidemiological Perspective
- Evaluation of Fatty Liver by Computed Tomography in Human