J Rhinol.
2021 Nov;28(3):180-185. 10.18787/jr.2021.00356.
Endonasal Septal Perforation Repair: Free Mucosal Graft With Lyoplant® Bioscaffold
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Jeju National University School of Medicine, Jeju, Republic of Korea
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- KMID: 2522789
- DOI: http://doi.org/10.18787/jr.2021.00356
Abstract
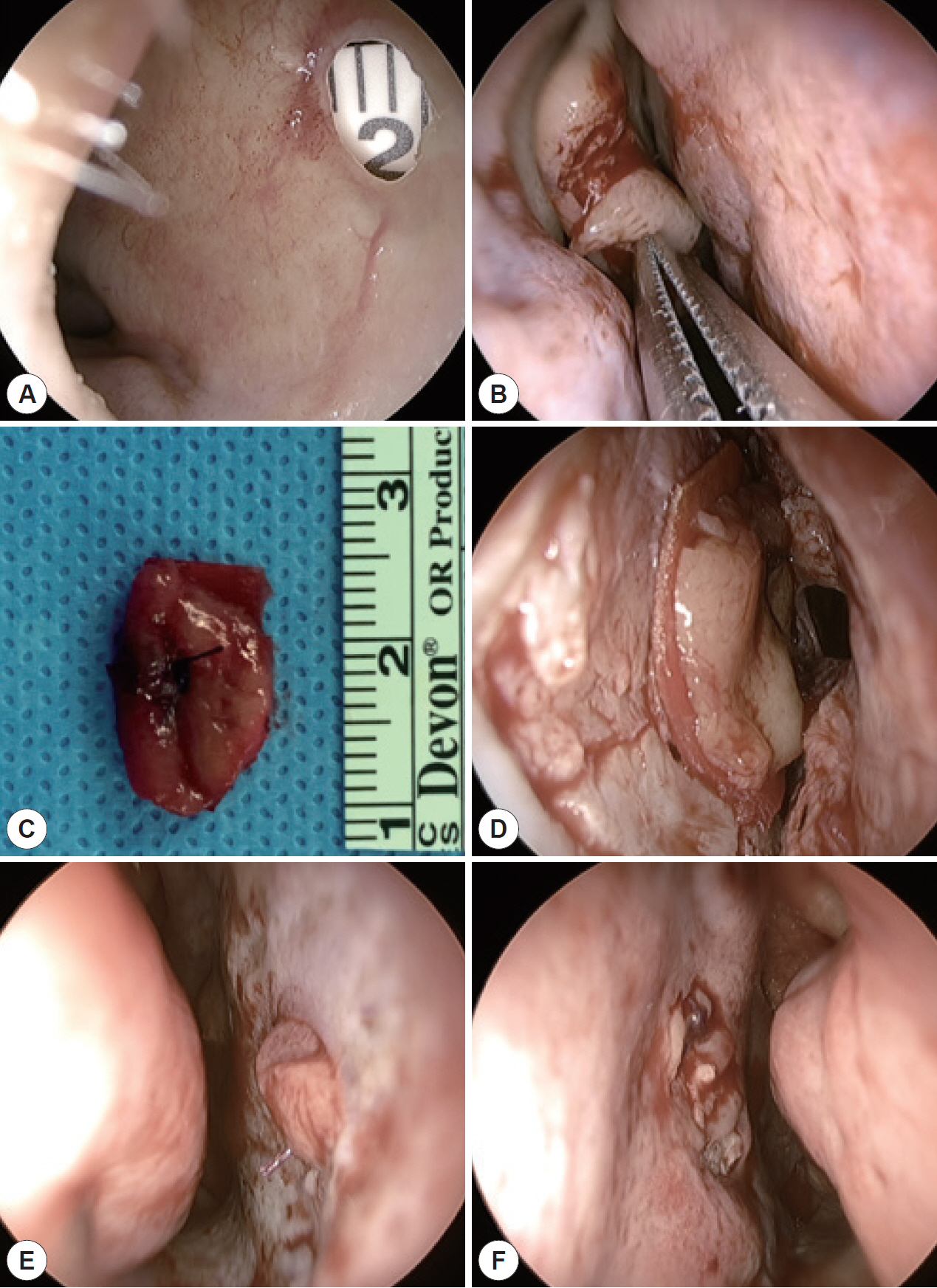
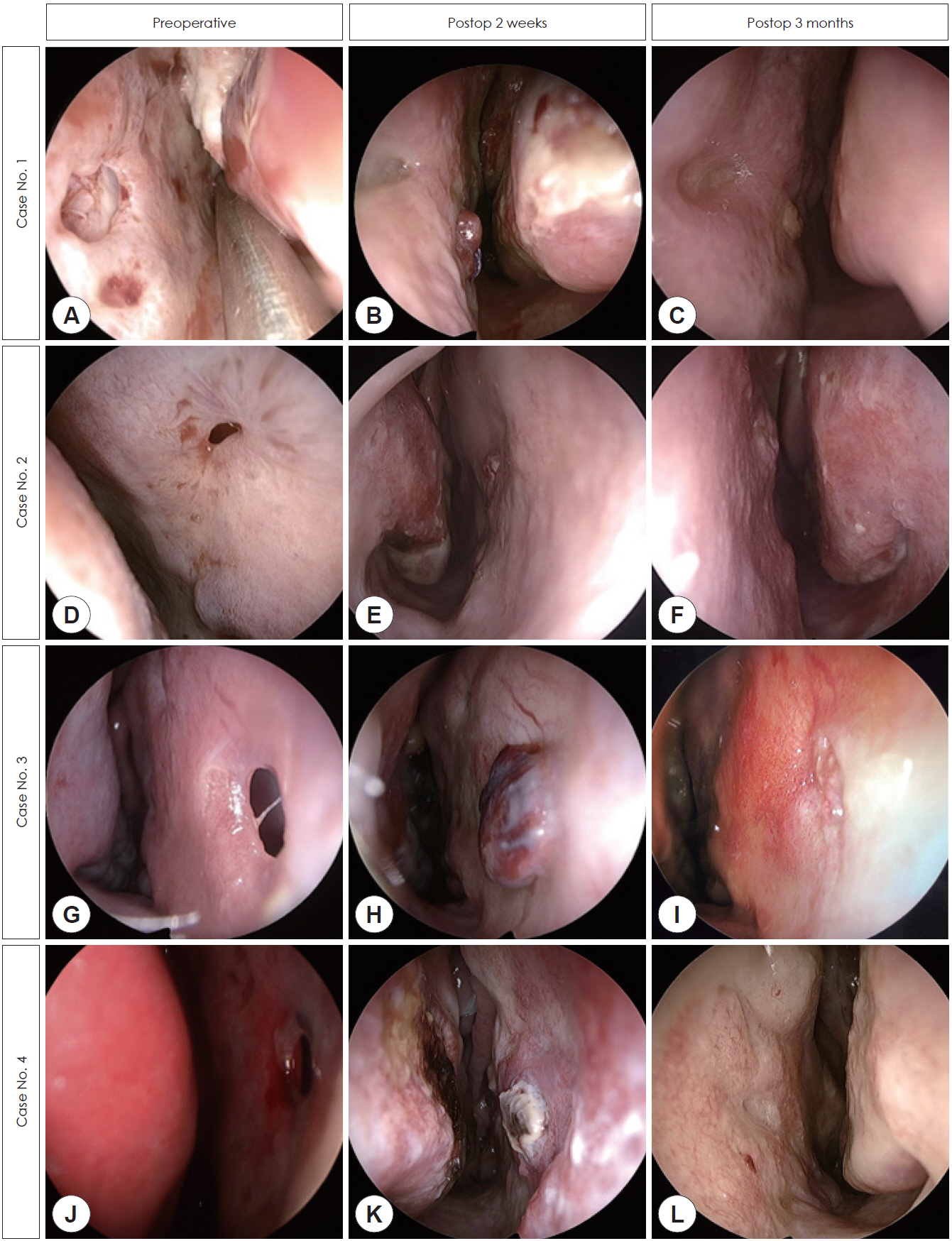
- Nasal septal perforation (NSP) is a common complication of nasal surgery and can cause nasal obstruction, crust, and epistaxis. Many surgical methods have been introduced for repair of NSP, among which mucosal flap and artificial dermis have been widely used. However, mucosal graft can shrink and migrate and is difficult to fix at the perforation site. Mucosal advancement flap requires a wide extent of septal mucosa dissection, and artificial dermis can cause nasal obstruction because of its bulkiness and lower biocompatibility than autologous mucosa. To overcome these problems, we reported successful outcomes in 4 cases of small NSP by free mucosal graft with bioscaffold.
Keyword
Figure
Reference
-
References
1. Hier MP, Yoskovitch A, Panje WR. Endoscopic repair of a nasal septal perforation. J Otolaryngol. 2002; 31(5):323–6.2. Lindemann J, Leiacker R, Stehmer V, Rettinger G, Keck T. Intranasal temperature and humidity profile in patients with nasal septal perforation before and after surgical closure. Clin Otolaryngol Allied Sci. 2001; 26(5):433–7.3. Goh AY, Hussain SS. Different surgical treatments for nasal septal perforation and their outcomes. J Laryngol Otol. 2007; 121(5):419–26.4. Mansour HA. Repair of nasal septal perforation using inferior turbinate graft. J Laryngol Otol. 2011; 125(5):474–8.5. Tastan E, Aydogan F, Aydin E, Can IH, Demirci M, Uzunkulaoglu H, et al. Inferior turbinate composite graft for repair of nasal septal perforation. Am J Rhinol Allergy. 2012; 26(3):237–42.6. Castelnuovo P, Ferreli F, Khodaei I, Palma P. Anterior ethmoidal artery septal flap for the management of septal perforation. Arch Facial Plast Surg. 2011; 13(6):411–4.7. Vuyk HD, Versluis RJ. The inferior turbinate flap for closure of septal perforations. Clin Otolaryngol Allied Sci. 1988; 13(1):53–7.8. Diamantopoulos II, Jones NS. The investigation of nasal septal perforations and ulcers. J Laryngol Otol. 2001; 115(7):541–4.9. Mobley SR, Boyd JB, Astor FC. Repair of a large septal perforation with a radial forearm free flap: brief report of a case. Ear Nose Throat J. 2001; 80(8):512.10. Ayshford CA, Shykhon M, Uppal HS, Wake M. Endoscopic repair of nasal septal perforation with acellular human dermal allograft and an inferior turbinate flap. Clin Otolaryngol Allied Sci. 2003; 28(1):29–33.11. Kim SW, Rhee CS. Nasal septal perforation repair: predictive factors and systematic review of the literature. Curr Opin Otolaryngol Head Neck Surg. 2012; 20(1):58–65.12. Murrell GL, Karakla DW, Messa A. Free flap repair of septal perforation. Plast Reconstr Surg. 1998; 102(3):818–21.13. Kridel RW, Foda H, Lunde KC. Septal perforation repair with acellular human dermal allograft. Arch Otolaryngol Head Neck Surg. 1998; 124(1):73–8.14. Winde F, Backhaus K, Zeitler JA, Schlegel N, Meyer T. Bladder augmentation using Lyoplant® : first experimental results in rats. Tissue Eng Regen Med. 2019; 16(6):645–52.15. Meyer T, Schwarz K, Ulrichs K, Höcht B. A new biocompatible material (Lyoplant®) for the therapy of congenital abdominal wall defects: first experimental results in rats. Pediatr Surg Int. 2006; 22(4):369–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Repair of Nasal Septal Perforation Using Polycaprolactone Plate and Temporalis Fascia Graft
- Repair of Nasal Septal Perforation Using Silastic Sheet
- Prevention Technique Using Inferior Turbinate Mucosal Flap for Septal Perforation after Septoplasty
- Sandwich Graft using Ear Cartilage and Inferior Turbinate Mucoperiosteal Free Graft Via Open Rhinoplasty Approach for Repair of Nasal Septal Perforation
- A Case of Septal Perforation Repair with Middle Turbinate Flap