Clin Endosc.
2021 Nov;54(6):825-832. 10.5946/ce.2021.048.
Endoscopic Evaluation of Biliary Strictures: Current and Emerging Techniques
- Affiliations
-
- 1Department of Upper Gastrointestinal Surgery, Concord Repatriation General Hospital, Sydney, NSW, Australia
- 2Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
- KMID: 2522702
- DOI: http://doi.org/10.5946/ce.2021.048
Abstract
- The diagnosis of biliary strictures in clinical practice can be challenging. Discriminating between benign and malignant biliary strictures is important to prevent the morbidity and mortality associated with incorrect diagnoses. Missing a malignant biliary stricture may delay surgery, resulting in poor prognostic outcomes. Conversely, it has been demonstrated that approximately 20% of patients who undergo surgery for suspected biliary malignancies have a benign etiology on histopathology. Traditional tissue sampling using endoscopic retrograde cholangiography does not always produce a definitive diagnosis, with a considerable proportion of cases remaining as indeterminate biliary strictures. Recent advances in endoscopic techniques have the potential to improve the diagnostic and prognostic accuracy of biliary strictures.
Figure
Reference
-
1. Dawwas MF, Oppong KW, Webster GJ. Endoscopic assessment and management of biliary strictures. Frontline Gastroenterol. 2016; 7:170–175.
Article2. Jha R, Al-Kawas FH. How good is IDUS in patients with isolated biliary strictures? A J Gastroenterol. 2004; 99:1690–1691.
Article3. Larghi A, Waxman I. Differentiating benign from malignant idiopathic biliary strictures: are we there yet? Gastrointest Endosc. 2007; 66:97–99.
Article4. Longmire WP, Macarthur MS, Bastounis EA, Hiatt J. Carcinoma of the extrahepatic biliary tract. Ann Surg. 1973; 178:333–345.
Article5. Gerhards MF, Vos P, Van Gulik TM, Rauws EA, Bosma A, Gouma DJ. Incidence of benign lesions in patients resected for suspicious hilar obstruction. British J Surg. 2001; 88:48–51.
Article6. Clayton RaE, Clarke DL, Currie EJ, Madhavan KK, Parks RW, Garden OJ. Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgeon. 2003; 1:32–38.
Article7. Wakai T, Shirai Y, Sakata J, et al. Clinicopathological features of benign biliary strictures masquerading as biliary malignancy. Am Surg. 2012; 78:1388–1391.
Article8. Hall JG, Pappas TN. Current management of biliary strictures. J Gastrointest Surg. 2004; 8:1098–1110.
Article9. Bowlus CL, Olson KA, Gershwin ME. Evaluation of indeterminate biliary strictures. Nat Rev Gastroenterol Hepatol. 2016; 13:28–37.
Article10. Martin RF, Rossi RL. Bile duct injuries: spectrum, mechanisms of injury, and their prevention. Surg Clin North Am. 1994; 74:781–803.
Article11. Kaya M, Petersen BT, Angulo P, et al. Balloon dilation compared to stenting of dominant strictures in primary sclerosing cholangitis. Am J Gastroenterol. 2001; 96:1059–1066.
Article12. Ohara H, Okazaki K, Tsubouchi H, et al. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012; 19:536–542.13. Alam I, Levenson SD, Ferrell LD, Bass NM. Diffuse intrahepatic biliary strictures in sarcoidosis resembling sclerosing cholangitis (case report and review of the literature). Dig Dis Sci. 1997; 42:1295–1301.14. Wong CS, Al-Ajami AK, Crotty JM, Naqvi SA. Benign biliary stricture and its rare association—Mirizzi syndrome: a case series and literature review. Int J Case Rep Imag. 2012; 3:1–7.15. Liberman E, Yen TS. Foamy macrophages in acquired immunodeficiency syndrome cholangiopathy with Encephalitozoon intestinalis. Arch Pathol Lab Med. 1997; 121:985–988.16. Chen XM, LaRusso NF. Cryptosporidiosis and the pathogenesis of AIDS-cholangiopathy. Semin Liver Dis. 2002; 22:277–290.
Article17. Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep. 2015; 3:22–31.
Article18. Xie C, Aloreidi K, Patel B, et al. Indeterminate biliary strictures: a simplified approach. Expert Rev Gastroenterol Hepatol. 2018; 12:189–199.
Article19. Salgado SM, Gaidhane M, Kahaleh M. Endoscopic palliation of malignant biliary strictures. World J Gastrointest Oncol. 2016; 8:240–247.
Article20. Stewart CJ, Mills PR, Carter R, et al. Brush cytology in the assessment of pancreatico–biliary strictures: a review of 406 cases. J Clin Pathol. 2001; 54:449–455.
Article21. Ödemiş B, Parlak E, Başar Ö, Yüksel O, Şahin B. Biliary tract obstruction secondary to malignant lymphoma: experience at a referral center. Dig Dis Sci. 2007; 52:2323–2332.
Article22. Kurzawinski TR, Deery A, Dooley JS, Dick R, Hobbs KE, Davidson BR. A prospective study of biliary cytology in 100 patients with bile duct strictures. Hepatology. 1993; 18:1399–1403.
Article23. Seo DW, Lee SK, Yoo KS, et al. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc. 2000; 52:630–634.
Article24. Uhlmann D, Wiedmann M, Schmidt F, et al. Management and outcome in patients with Klatskin-mimicking lesions of the biliary tree. J Gastrointest Surg. 2006; 10:1144–1150.
Article25. Paranandi B, Oppong KW. Biliary strictures: endoscopic assessment and management. Frontline Gastroenterol. 2017; Apr. 1. 8:133–137.
Article26. Adler DG, Baron TH, Davila RE, et al. ASGE guideline: the role of ERCP in diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2005; 62:1–8.
Article27. Moon YM, Kim WH, Shin ST, et al. A study of 122 cases of pancreatic cancer diagnosed by endoscopic retrograde cholangiopancreatography (ERCP). Korean J Intern Med. 1986; 1:131–139.28. Park MS, Kim TK, Kim KW, et al. Differentiation of extrahepatic bile duct cholangiocarcinoma from benign stricture: findings at MRCP versus ERCP. Radiology. 2004; 233:234–240.
Article29. Kalaitzakis E, Levy M, Kamisawa T, et al. Endoscopic retrograde cholangiography does not reliably distinguish IgG4-associated cholangitis from primary sclerosing cholangitis or cholangiocarcinoma. Clin Gastroenterol Hepatol. 2011; 9:800–803.
Article30. Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013; 184:304–311.31. Trikudanathan G, Navaneethan U, Njei B, Vargo JJ, Parsi MA. Diagnostic yield of bile duct brushings for cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014; 79:783–789.32. Kitajima Y, Ohara H, Nakazawa T, et al. Usefulness of transpapillary bile duct brushing cytology and forceps biopsy for improved diagnosis in patients with biliary strictures. J Gastroenterol Hepatol. 2007; 22:1615–1620.
Article33. Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015; 81:168–176.
Article34. Athanassiadou P, Grapsa D. Value of endoscopic retrograde cholangiopancreatography-guided brushings in preoperative assessment of pancreaticobiliary strictures: what’s new? Acta Cytol. 2008; 52:24–34.35. Kurzawinski T, Deery A, Davidson BR. Diagnostic value of cytology for biliary stricture. Br J Surg. 1993; 80:414–421.
Article36. Rupp M, Hawthorne CM, Ehya H. Brushing cytology in biliary tract obstruction. Acta Cytol. 1990; 34:221–226.37. Pugliese V, Conio M, Nicolò G, Saccomanno S, Gatteschi B. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc. 1995; 42:520–526.
Article38. Okonkwo AM, De Frias DVS, Gunn R, et al. Reclassification of ”atypical” diagnoses in endoscopic retrograde cholangiopancreaticography-guided biliary brushings. Acta Cytol. 2003; 47:435–442.
Article39. De Bellis M, Fogel EL, Sherman S, et al. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc. 2003; 58:176–182.
Article40. Fogel EL, De Bellis M, McHenry L, et al. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc. 2006; 63:71–77.
Article41. Mishra G, Conway JD. Endoscopic ultrasound in the evaluation of radiologic abnormalities of the liver and biliary tree. Curr Gastroenterol Rep. 2009; 11:150–154.
Article42. Chung A, Kwan V. Endoscopic ultrasound: an overview of its role in current clinical practice. Australas J Ultrasound Med. 2009; 12:21–29.
Article43. Conway JD, Mishra G. The role of endoscopic ultrasound in biliary strictures. Curr Gastroenterol Rep. 2008; 10:157–162.
Article44. Fritscher-Ravens A, Broering DC, Sriram PV, et al. EUS-guided fine-needle aspiration cytodiagnosis of hilar cholangiocarcinoma: a case series. Gastrointest Endosc. 2000; 52:534–540.
Article45. Sadeghi A, Mohamadnejad M, Islami F, et al. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc. 2016; 83:290–298.
Article46. Ge N, Zhang S, Jin Z, et al. Clinical use of endoscopic ultrasound-guided fine-needle aspiration: guidelines and recommendations from Chinese Society of Digestive Endoscopy. Endosc Ultrasound. 2017; 6:75–82.
Article47. Levy MJ, Wiersema MJ. EUS-guided trucut biopsy. Gastrointest Endosc. 2005; 62:417–426.
Article48. Rodrigues-Pinto E, Grimm IS, Baron TH. Endoscopic ultrasound fine-needle aspiration vs. fine-needle biopsy: tissue is always the issue. Endosc Int Open. 2016; 4:E506–E507.
Article49. Aadam AA, Wani S, Amick A, et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016; 4:E497–E505.
Article50. Cosgrove ND, Yan L, Siddiqui A. Preoperative endoscopic ultrasound-guided fine needle aspiration for diagnosis of pancreatic cancer in potentially resectable patients: Is this safe? Endosc Ultrasound. 2015; 4:81–84.
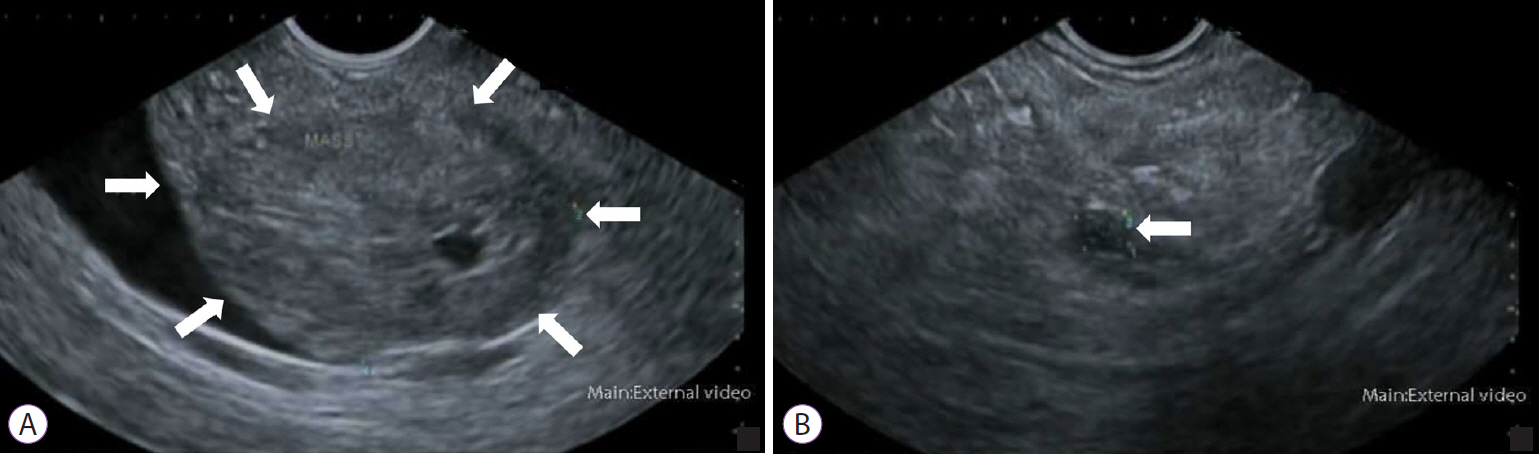
Article51. Vazquez-Sequeiros E, Baron TH, Clain JE, et al. Evaluation of indeterminate bile duct strictures by intraductal US. Gastrointest Endosc. 2002; 56:372–379.
Article52. Domagk D, Poremba C, Dietl KH, et al. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study. Gut. 2002; 51:240–244.
Article53. Farrell RJ, Agarwal B, Brandwein SL, Underhill J, Chuttani R, Pleskow DK. Intraductal US is a useful adjunct to ERCP for distinguishing malignant from benign biliary strictures. Gastrointest Endosc. 2002; 56:681–687.
Article54. Menzel J, Domschke W. Intraductal ultrasonography (IDUS) of the pancreato-biliary duct system: personal experience and review of literature. Eir J Ultrasound. 1999; 10:105–115.55. Tamada K, Tomiyama T, Wada S, et al. Endoscopic transpapillary bile duct biopsy with the combination of intraductal ultrasonography in the diagnosis of biliary strictures. Gut. 2002; 50:326–331.
Article56. Heinzow HS, Kammerer S, Rammes C, Wessling J, Domagk D, Meister T. Comparative analysis of ERCP, IDUS, EUS and CT in predicting malignant bile duct strictures. World J Gastroenterol. 2014; 20:10495–10503.
Article57. Kim HS, Moon JH, Lee YN, et al. Prospective comparison of intraductal ultrasonography-guided transpapillary biopsy and conventional biopsy on fluoroscopy in suspected malignant biliary strictures. Gut Liver. 2018; 12:463–470.
Article58. Menzel J, Hoepffner N, Sulkowski U, et al. Polypoid tumors of the major duodenal papilla: preoperative staging with intraductal US, EUS, and CT—a prospective, histopathologically controlled study. Gastrointest Endosc. 1999; 49:349–357.59. Menzel J, Poremba C, Dietl KH, Domschke W. Preoperative diagnosis of bile duct strictures--comparison of intraductal ultrasonography with conventional endosonography. Scand J Gastroenterol. 2000; 35:77–82.60. Rizvi S, Eaton J, Yang JD, Chandrasekhara V, Gores GJ. Emerging technologies for the diagnosis of perihilar cholangiocarcinoma. Semin Liver Dis. 2018; 38:160–169.
Article61. Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004; 99:1069–1073.
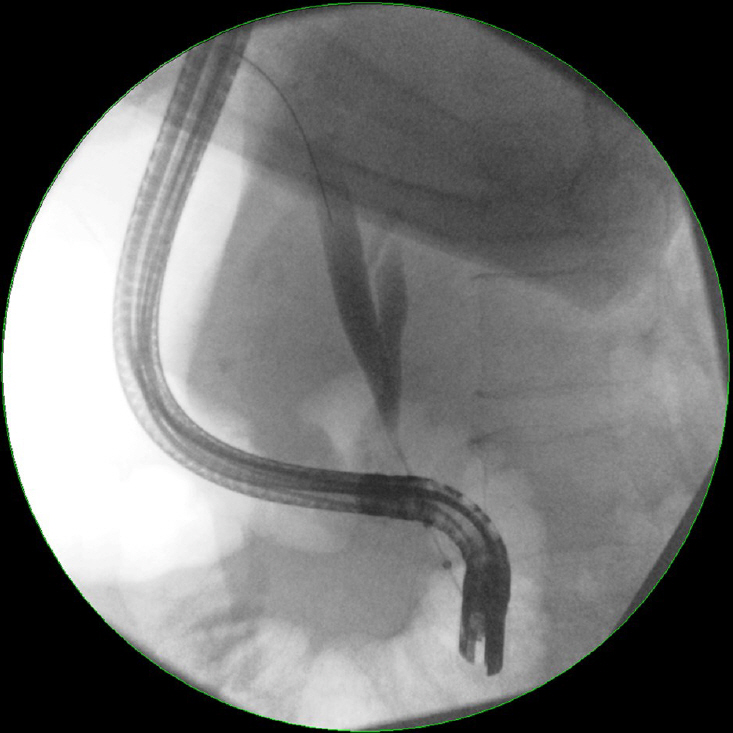
Article62. Monga A, Ramchandani M, Reddy DN. Per-oral cholangioscopy. J Interv Gastroenterol. 2011; 1:70–77.
Article63. Kim HJ, Kim MH, Lee SK, Yoo KS, Seo DW, Min YI. Tumor vessel: a valuable cholangioscopic clue of malignant biliary stricture. Gastrointest Endosc. 2000; 52:635–638.
Article64. Nimura Y, Kamiya J, Hayakawa N, Shionoya S. Cholangioscopic differentiation of biliary strictures and polyps. Endoscopy. 1989; 21:351–356.
Article65. Sun X, Zhou Z, Tian J, et al. Is single-operator peroral cholangioscopy a useful tool for the diagnosis of indeterminate biliary lesion? A systematic review and meta-analysis. Gastrointest Endosc. 2015; 82:79–87.
Article66. Shah RJ, Neuhaus H, Parsi M, Reddy DN, Pleskow DK. Randomized study of digital single-operator cholangioscope compared to fiberoptic single-operator cholangioscope in a novel cholangioscopy bench model. Endosc Int Open. 2018; 6:E851–E858.
Article67. Draganov P. The SpyGlass® direct visualization system for cholangioscopy. Gastroenterol Hepatol. 2008; 4:469–470.68. Bernica J, Elhanafi S, Kalakota N, et al. Cholangioscopy is safe and feasible in elderly patients. Clin Gastroenterol Hepatol. 2018; 16:1293–1299.
Article69. Wong JC, Tang RS, Teoh AY, Sung JJ, Lau JY. Efficacy and safety of novel digital single-operator peroral cholangioscopy-guided laser lithotripsy for complicated biliary stones. Endosc Int Open. 2017; 5:E54–E58.70. Shieh FK, Drumm H, Nathanson MH, Jamidar PA. High-definition confocal endomicroscopy of the common bile duct. J Clin Gastroenterol. 2012; 46:401–406.
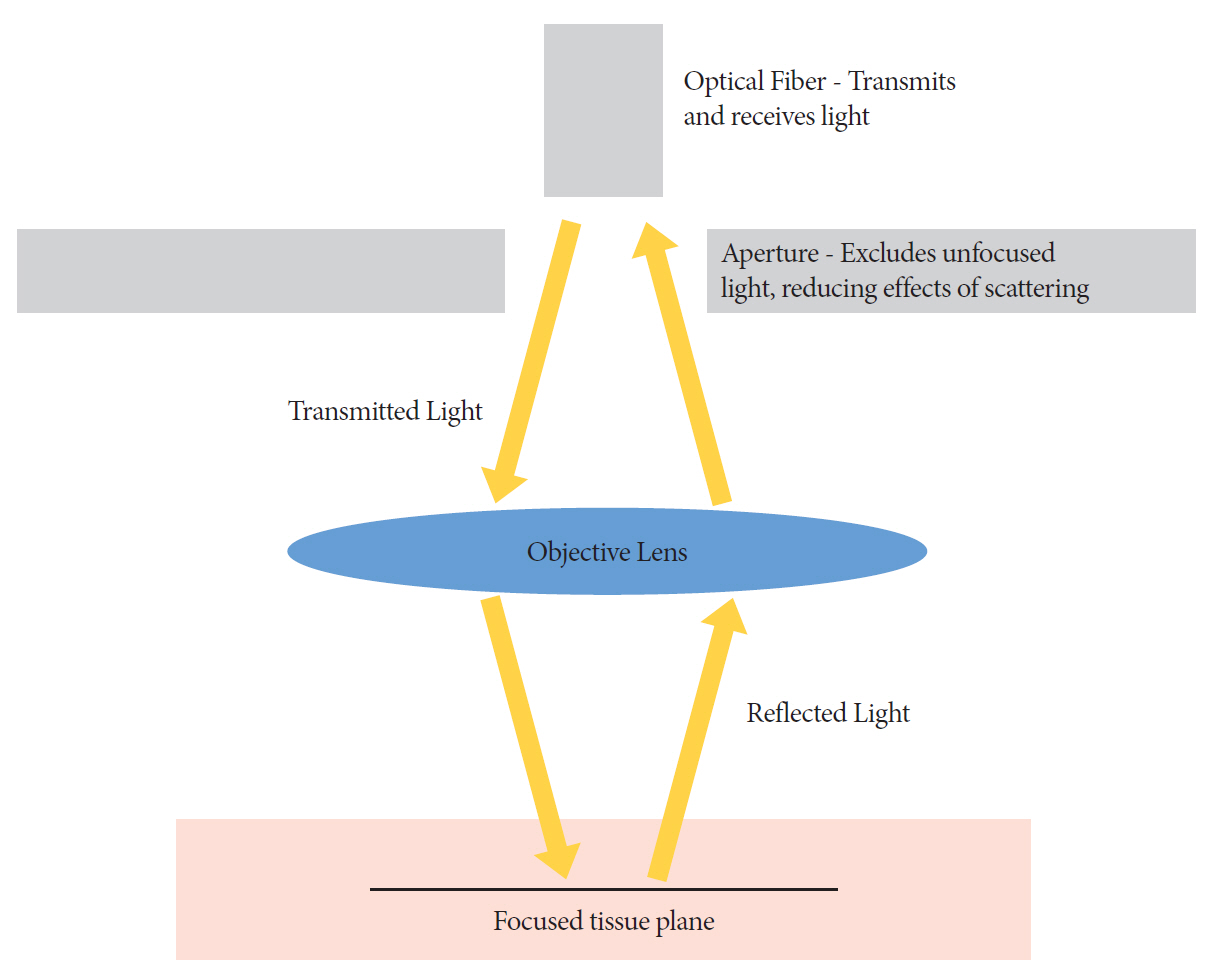
Article71. Polglase AL, McLaren WJ, Delaney PM. Pentax confocal endomicroscope: a novel imaging device for in vivo histology of the upper and lower gastrointestinal tract. Expert Rev Med Devices. 2006; 3:549–556.72. Meining A, Saur D, Bajbouj M, et al. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: an examiner blinded analysis. Clin Gastroenterol Hepatol. 2007; 5:1261–1267.
Article73. De Palma GD. Confocal laser endomicroscopy in the “in vivo” histological diagnosis of the gastrointestinal tract. World J Gastroenterol. 2009; 15:5770–5775.74. Meining A, Shah RJ, Slivka A, et al. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy. 2012; 44:251–257.
Article75. Meining A, Chen YK, Pleskow D, et al. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc. 2011; 74:961–968.
Article76. Caillol F, Filoche B, Gaidhane M, Kahaleh M. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris classification. Dig Dis Sci. 2013; 58:1784–1789.
Article77. Gao YD, Qu YW, Liu HF. Comparison of diagnostic efficacy between CLE, tissue sampling, and CLE combined with tissue sampling for undetermined pancreaticobiliary strictures: a meta-analysis. Scand J Gastroenterol. 2018; 53:482–489.
Article78. Talreja JP, Sethi A, Jamidar PA, et al. Interpretation of probe-based confocal laser endomicroscopy of indeterminate biliary strictures: is there any interobserver agreement? Dig Dis Sci. 2012; 57:3299–3302.
Article79. Talreja JP, Turner BG, Gress FG, et al. Pre‐and post‐training session evaluation for interobserver agreement and diagnostic accuracy of probe‐based confocal laser endomicroscopy for biliary strictures. Dig Endosc. 2014; 26:577–580.80. Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol. 2008; 26:646–651.
Article81. Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006; 131:1064–1072.
Article82. Fritcher EG, Kipp BR, Halling KC, et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology. 2009; 136:2180–2186.
Article83. Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004; 99:1675–1681.
Article84. Smoczynski M, Jablonska A, Matyskiel A, et al. Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures. Gastrointest Endosc. 2012; 75:65–73.
Article85. Chaiteerakij R, Barr Fritcher EG, Angsuwatcharakon P, et al. Fluorescence in situ hybridization compared with conventional cytology for the diagnosis of malignant biliary tract strictures in Asian patients. Gastrointest Endosc. 2016; 83:1228–1235.
Article86. Wu X, Zeng X, Yang A, et al. Fluorescence in situ hybridization with the urovysion kit for the detection of biliary cancer in Chinese patients. Clin Lab. 2017; 63:407–413.
Article87. Liew ZH, Loh TJ, Lim TKH, et al. Role of fluorescence in situ hybridization in diagnosing cholangiocarcinoma in indeterminate biliary strictures. J Gastroenterol Hepatol. 2018; 33:315–319.
Article88. Menias CO, Surabhi VR, Prasad SR, Wang HL, Narra VR, Chintapalli KN. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics. 2008; 28:1115–1129.
Article89. Takakura WR, Tabibian JH, Bowlus CL. The evolution of natural history of primary sclerosing cholangitis. Curr Opin Gastroenterol. 2017; 33:71–77.
Article90. Navaneethan U, Njei B, Venkatesh PG, Vargo JJ, Parsi MA. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc. 2014; 79:943–950.
Article91. Bangarulingam SY, Bjornsson E, Enders F, et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology. 2010; 51:174–180.
Article92. Testoni PA, Mangiavillano B, Mariani A. Optical coherence tomography for investigation of the pancreatico-biliary system: still experimental? JOP. 2007; 8:156–165.93. Testoni PA, Mangiavillano B. Optical coherence tomography for bile and pancreatic duct imaging. Gastrointest Endosc Clin N Am. 2009; 19:637–653.
Article94. Testoni PA, Mangiavillano B. Optical coherence tomography in detection of dysplasia and cancer of the gastrointestinal tract and bilio-pancreatic ductal system. World J Gastroenterol. 2008; 14:6444–6452.
Article95. Singh P, Chak A, Willis JE, Rollins A, Sivak Jr MV. In vivo optical coherence tomography imaging of the pancreatic and biliary ductal system. Gastrointest Endosc. 2005; 62:970–974.
Article96. Testoni PA, Mariani A, Mangiavillano B, et al. Main pancreatic duct, common bile duct and sphincter of Oddi structure visualized by optical coherence tomography: An ex vivo study compared with histology. Dig Liver Dis. 2006; 38:409–414.
Article97. Seitz U, Freund J, Jaeckle S, et al. First in vivo optical coherence tomography in the human bile duct. Endoscopy. 2001; 33:1018–1021.
Article98. Poneros JM, Tearney GJ, Shiskov M, et al. Optical coherence tomography of the biliary tree during ERCP. Gastrointest Endosc. 2002; 55:84–88.
Article99. Arvanitakis M, Hookey L, Tessier G, et al. Intraductal optical coherence tomography during endoscopic retrograde cholangiopancreatography for investigation of biliary strictures. Endoscopy. 2009; 41:696–701.
Article100. De Boer JF, Leitgeb R, Wojtkowski M. Twenty-five years of optical coherence tomography: the paradigm shift in sensitivity and speed provided by Fourier domain OCT. Biomed Opt Express. 2017; 8:3248–3280.101. Tyberg A, Xu MM, Gaidhane M, Kahaleh M. Second generation optical coherence tomography: preliminary experience in pancreatic and biliary strictures. Dig Liver Dis. 2018; 50:1214–1217.
Article102. Yoon WJ, Brugge WR. Endoscopic evaluation of bile duct strictures. Gastrointest Endosc Clin N Am. 2013; 23:277–293.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent advances of diagnostic approaches for indeterminate biliary tract obstruction
- Diagnostic Approach to Indeterminate Biliary Stricture
- Percutaneous intervention for bilioenteric anastomotic strictures: Current strategies and future directions
- Endoscopic Diagnosis and Treatment of Benign Biliary Strictures
- Endoscopic Ultrasonography in the Evaluation of Indeterminate Biliary Strictures