Arch Hand Microsurg.
2021 Dec;26(4):285-292. 10.12790/ahm.21.0118.
Hemodynamic Principles in Free Tissue Transfer: Vascular Changes at the Anastomosis Site
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Dongguk University School of Medicine, Seoul, Korea
- KMID: 2522627
- DOI: http://doi.org/10.12790/ahm.21.0118
Abstract
- Purpose
Various factors such as blood velocity, turbulent flow,and intimal injury are the most basic elements in free tissue transfers. However, how blood flow is reestablished, maintained, and changed after vascular anastomosis has rarely been studied.
Methods
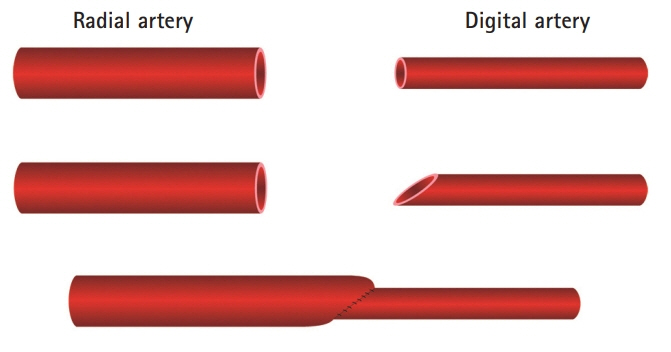
A 54-year-old male sustained an unreplantable severe crushing injury to his right hand. The middle finger was transferred to the thumb as an ectopic replantation using an anastomosis between the radial and digital arteries. However, secondary reconstruction for the first web space defect was inevitable and an anteromedial thigh free flap procedure was performed 2 months later using the previously anastomosed vessels. During the procedures, we noted morphologic changes in the microvessels and tried to explain those phenomena by applying the principles of hemodynamics.
Results
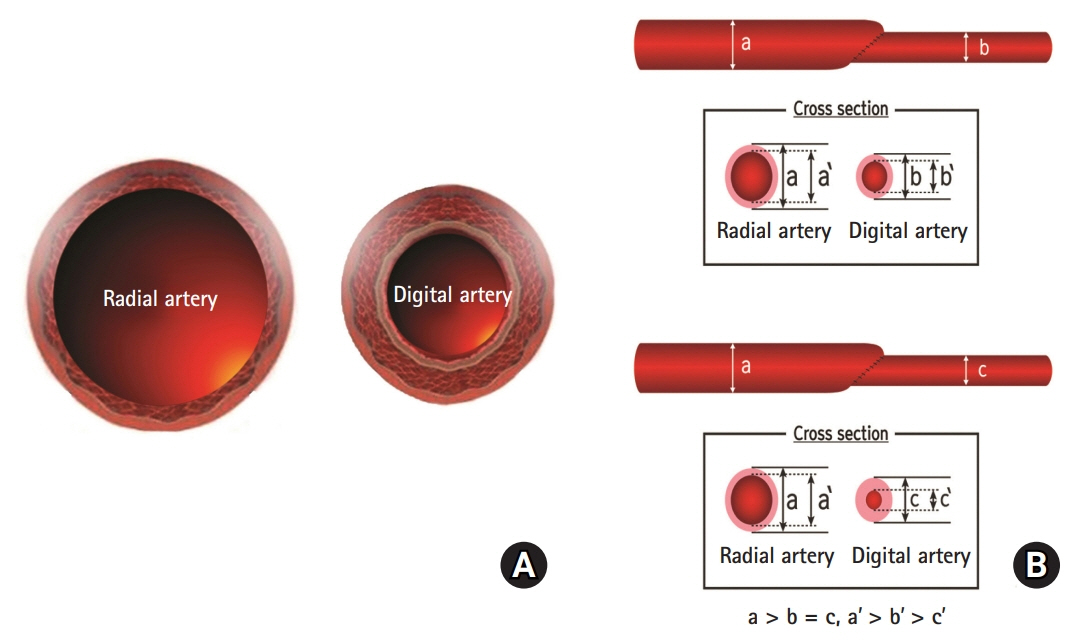
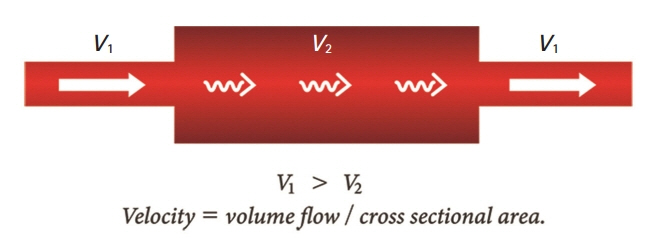
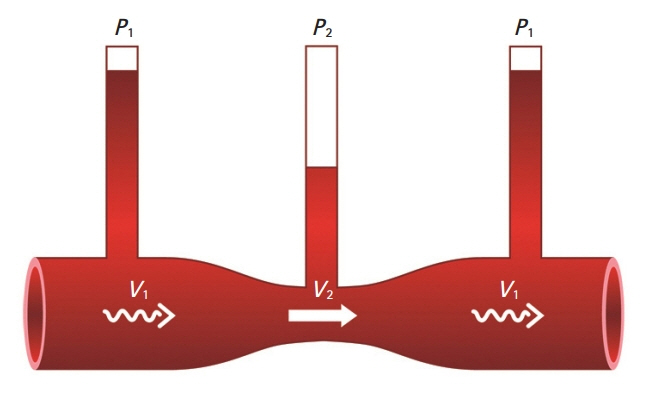
Due to the discrepancy in vascular size between the radial and digital arteries, the velocity of the blood flow in the post-anastomotic site, which was the digital artery, must have been increased by Poiseuille’s law. Supposing that the velocity through the post-anastomotic site of the digital artery was increased, the pressure exerted by that flow decreased, resulting in more shrinkage of the vessel lumen of the digital artery by Bernoulli’s principle. Pascal’s law could also be applied in confined spaces with a static flow; where there is a constant pressure, as the radius of the post-anastomotic digital artery diminishes, the tension within the digital artery’s wall also simultaneously decreases. By Laplace’s law, the post-anastomotic digital artery’s wall thickens as less tension is exerted on the wall.
Conclusion
Understanding these simple flow mechanics will enable microsurgeons to better avoid the risk factors causing thrombosis, which is related to flap failure.
Keyword
Figure
Reference
-
1. Bui DT, Cordeiro PG, Hu QY, Disa JJ, Pusic A, Mehrara BJ. Free flap reexploration: indications, treatment, and outcomes in 1193 free flaps. Plast Reconstr Surg. 2007; 119:2092–100.
Article2. Sigurdsson GH. Perioperative fluid management in microvascular surgery. J Reconstr Microsurg. 1995; 11:57–65.
Article3. Booi DI. Perioperative fluid overload increases anastomosis thrombosis in the free TRAM flap used for breast reconstruction. Eur J Plast Surg. 2011; 34:81–6.
Article4. Lorenzetti F, Salmi A, Ahovuo J, Tukiainen E, Asko-Seljavaara S. Postoperative changes in blood flow in free muscle flaps: a prospective study. Microsurgery. 1999; 19:196–9.
Article5. Ichinose A, Tahara S, Terashi H, Yokoo S. Reestablished circulation after free radial forearm flap transfer. J Reconstr Microsurg. 2004; 20:207–13.
Article6. Numata T, Iida Y, Shiba K, et al. Usefulness of color Doppler sonography for assessing hemodynamics of free flaps for head and neck reconstruction. Ann Plast Surg. 2002; 48:607–12.
Article7. Hanasono MM, Ogunleye O, Yang JS, Hartley CJ, Miller MJ. Changes in blood velocity following microvascular free tissue transfer. J Reconstr Microsurg. 2009; 25:417–24.
Article8. Lorenzetti F, Ahovuo J, Suominen S, Salmi A, Asko-Seljavaara S. Colour Doppler ultrasound evaluation of haemodynamic changes in free tram flaps and their donor sites. Scand J Plast Reconstr Surg Hand Surg. 2002; 36:202–6.
Article9. Bodor R, Yoleri L, Zhang F, Buncke GM, Lineaweaver WC, Buncke HJ. Blood-flow velocity as a factor in postoperative microvascular patency. J Reconstr Microsurg. 1997; 13:463–70.
Article10. Szilagyi DE, Whitcomb JG, Schenker W, Waibel P. The laws of fluid flow and arterial grafting. Surgery. 1960; 46:55–73.11. Miyamoto S, Takushima A, Okazaki M, Ohura N, Minabe T, Harii K. Relationship between microvascular arterial anastomotic type and area of free flap survival: comparison of end-to-end, end-to-side, and retrograde arterial anastomosis. Plast Reconstr Surg. 2008; 121:1901–8.
Article12. Yoon AP, Jones NF. Critical time for neovascularization/angiogenesis to allow free flap survival after delayed postoperative anastomotic compromise without surgical intervention: a review of the literature. Microsurgery. 2016; 36:604–12.
Article13. Seidenberg B, Hurwitt ES, Carton CA. The technique of anastomosing small arteries. Surg Gynecol Obstet. 1958; 106:743–6.14. O’Brien BM, Morrison WA, Ishida H, MacLeod AM, Gilbert A. Free flap transfers with microvascular anastomoses. Br J Plast Surg. 1974; 27:220–30.15. Monsivais JJ. Microvascular grafts: effect of diameter discrepancy on patency rates. Microsurgery. 1990; 11:285–7.
Article16. Fieldman JS, Phong DH, Saint-Aubin Y, Vinet L, editors. Biology and mechanics of blood flows, part II: mechanics and medical aspects. New York: Springer Science+Business Media LLC;2007. Chapter 4-2, Blood rheology; p. 119-22.17. Taylor KJ, Holland S. Doppler US. Part I. Basic principles, instrumentation, and pitfalls. Radiology. 1990; 174:297–307.
Article18. Zhang F, Oliva A, Kao SD, Newlin L, Buncke HJ. Microvascular vein grafts in the rat cutaneous free-flap model. J Reconstr Microsurg. 1994; 10:229–33.
Article19. Bayramiçli M, Tetik C, Sönmez A, Gürünlüoğlu R, Baltaci F. Reliability of primary vein grafts in lower extremity free tissue transfers. Ann Plast Surg. 2002; 48:21–9.20. Darius O. The clipped microvascular anastomosis: hemodynamical, morphological and surgical evaluation. Belgium: Leuven University Press;2004.21. del Zoppo GJ. Virchow’s triad: the vascular basis of cerebral injury. Rev Neurol Dis. 2008; 5(Suppl 1):S12–21.22. Allan BD. Mechanism of iris prolapse: a qualitative analysis and implications for surgical technique. J Cataract Refract Surg. 1995; 21:182–6.
Article23. Beris AE, Soucacos PN, Touliatos AS. Experimental evaluation of the length of microvenous grafts under normal tension. Microsurgery. 1992; 13:195–9.
Article24. Recordati G. The contribution of the giraffe to hemodynamic knowledge: a unified physical principle for the circulation. Cardiologia. 1999; 44:783–9.25. Chen KT, Mardini S, Chuang DC, et al. Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast Reconstr Surg. 2007; 120:187–95.
Article26. Siemionow M, Andreasen T, Chick L, Lister G. Effect of muscle flap denervation on flow hemodynamics: a new model for chronic in vivo studies. Microsurgery. 1994; 15:891–4.
Article27. Lorenzetti F, Giordano S, Tukiainen E. Intraoperative hemodynamic evaluation of the latissimus dorsi muscle flap: a prospective study. J Reconstr Microsurg. 2012; 28:273–8.
Article28. Chen LE, Seaber AV, Bossen E, Urbaniak JR. The effect of acute denervation on the microcirculation of skeletal muscle: rat cremaster model. J Orthop Res. 1991; 9:266–74.
Article29. Nasir S, Baykal B, Altuntaş S, Aydin MA. Hemodynamic differences in blood flow between free skin and muscles flaps: prospective study. J Reconstr Microsurg. 2009; 25:355–60.
Article30. Kurita M, Takushima A, Shiraishi T, Okazaki M, Ozaki M, Harii K. Alteration of arterial blood flow after free muscle transfer. Ann Plast Surg. 2010; 64:477–81.
Article31. Lorenzetti F, Giordano S, Suominen E, Asko-Seljavaara S, Suominen S. Intraoperative hemodynamic evaluation of the radial and ulnar arteries during free radial forearm flap procedure. J Reconstr Microsurg. 2010; 26:73–7.
Article32. Lorenzetti F, Kuokkanen H, von Smitten K, Asko-Seljavaara S. Intraoperative evaluation of blood flow in the internal mammary or thoracodorsal artery as a recipient vessel for a free TRAM flap. Ann Plast Surg. 2001; 46:590–3.
Article33. Nasir S, Aydin MA, Sonmez E, Baykal B. Flow-through free latissimus dorsi flap for reconstruction of injured limbs: evaluation of hemodynamic effects on extremity circulation. Ann Plast Surg. 2010; 65:164–9.34. Cooley BC, Lan M, Gould JS. Rat femoral vein-to-vein grafts as a microvascular practice model: factors that influence patency. Microsurgery. 1991; 12:43–5.
Article35. Buncke HJ, Alpert B, Shah KG. Microvascular grafting. Clin Plast Surg. 1978; 5:185–94.
Article36. Fujikawa S, O'Brien BM. An experimental evaluation of microvenous grafts. Br J Plast Surg. 1975; 28:244–6.
Article37. Salgado CJ, Smith A, Kim S, et al. Effects of late loss of arterial inflow on free flap survival. J Reconstr Microsurg. 2002; 18:579–84.
Article38. Lee CH, Han SK, Dhong ES, Kim HP, Kim WK. The fate of microanastomosed digital arteries after successful replantation. Plast Reconstr Surg. 2005; 116:805–10.
Article39. Lorenzetti F, Suominen S, Tukiainen E, et al. Evaluation of blood flow in free microvascular flaps. J Reconstr Microsurg. 2001; 17:163–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Recipient Vessel in Free Tissue Transfer to Lower Extremity
- Risk and Effectiveness of Using Thrombin in Microvascular Free Tissue Transfer
- Osteocutaneous Free Flap Transfer by Microsurgical Technique
- The Experimental Investigation for the Vascularizaion of Free Flap in Rats
- Chinical Analysis of Lower Leg Reconstruction with Free Flaps (47 Cases)