Obstet Gynecol Sci.
2021 Nov;64(6):524-531. 10.5468/ogs.21176.
Prognostic significance of tumor laterality in advanced ovarian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Nara Medical University, Kashihara, Japan
- 2Department of Gynecology, Osaka International Cancer Institute, Osaka, Japan
- KMID: 2522476
- DOI: http://doi.org/10.5468/ogs.21176
Abstract
Objective
This study aimed to investigate the effect of incorporating tumor laterality into the International Federation of Gynecology and Obstetrics (FIGO) staging system for advanced ovarian cancer.
Methods
The clinical data of 131 patients with advanced ovarian cancer treated between 2008 and 2018 were retrospectively reviewed. To investigate the prognostic significance of tumor laterality, we divided the patients into unilateral and bilateral groups. The prognostic significance of tumor laterality (bilateral vs. unilateral) was evaluated using univariate and multivariate analyses. The effect of incorporating tumor laterality into the FIGO staging system to predict survival outcomes was evaluated using the Kaplan-Meier method.
Results
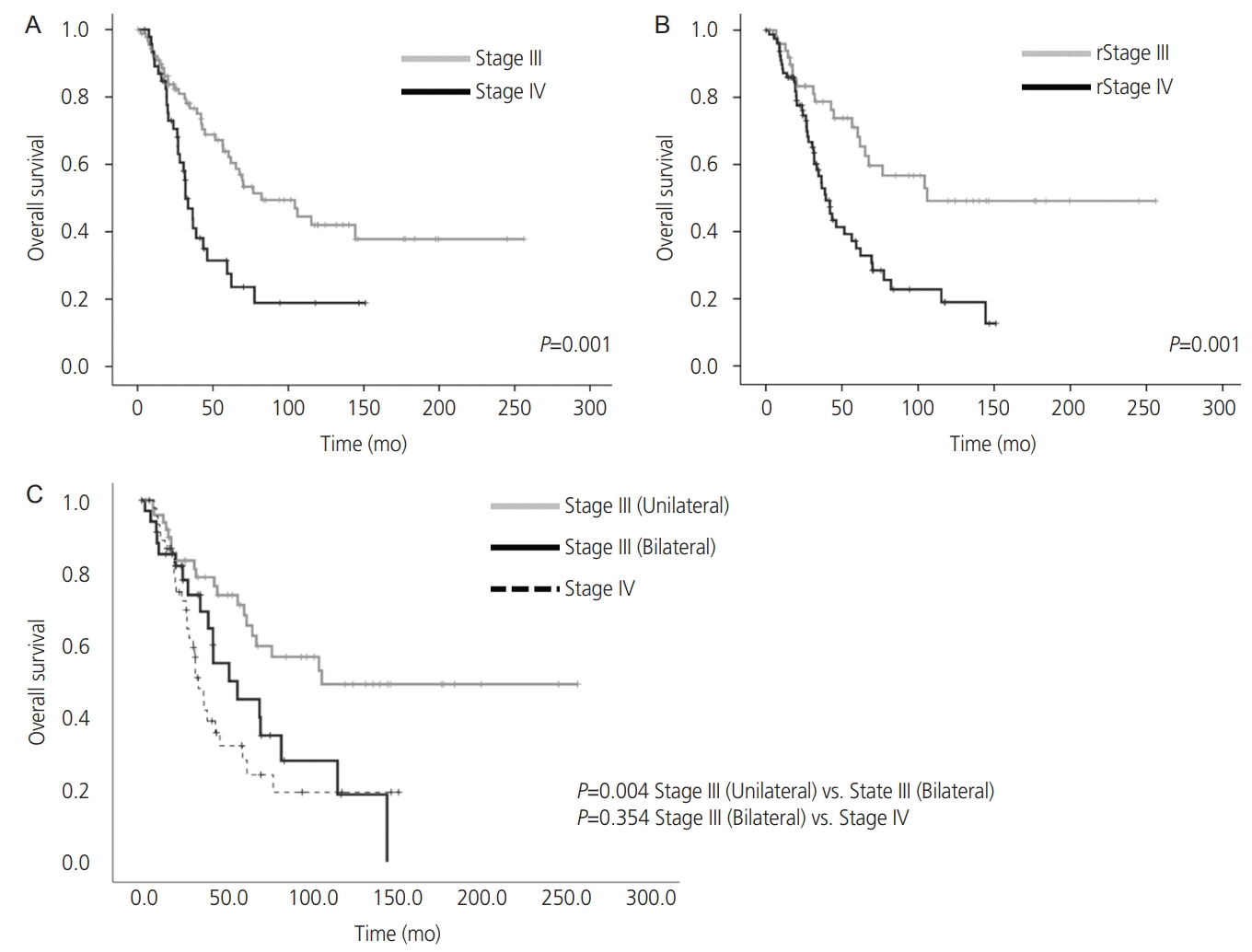
Both overall survival (OS) and progression-free survival (PFS) were longer in the unilateral group than in the bilateral group. Multivariate analysis demonstrated that tumor laterality was an independent predictor of OS (hazard ratio, 1.75; confidence interval, 1.05-2.92; P=0.032). In patients with stage III disease, the bilateral group had a shorter OS than the unilateral group, but it was comparable to the OS in stage IV patients (P=0.354). The incorporation of tumor laterality into the FIGO staging system improved the stratification of survival probabilities.
Conclusion
Tumor laterality can be an independent prognostic factor in patients with advanced ovarian cancer. The incorporation of tumor laterality may improve the predictive performance of the FIGO staging system in patients with advanced ovarian cancer.
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018; 68:284–96.
Article3. Tan DS, Rothermundt C, Thomas K, Bancroft E, Eeles R, Shanley S, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations”. J Clin Oncol. 2008; 26:5530–6.4. Gallagher DJ, Konner JA, Bell-McGuinn KM, Bhatia J, Sabbatini P, Aghajanian CA, et al. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011; 22:1127–32.
Article5. Negri F, De Giorgi A, Gilli A, Azzoni C, Bottarelli L, Gnetti L, et al. Impact of laterality and mucinous histology on relapse-free and overall survival in a registry-based colon cancer series. Sci Rep. 2019; 9:3668.
Article6. Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg. 2016; 20:648–55.
Article7. Brulé SY, Jonker DJ, Karapetis CS, O’Callaghan CJ, Moore MJ, Wong R, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015; 51:1405–14.
Article8. Barbara RC, Piotr R, Kornel B, Elżbieta Z, Danuta R, Eduardo N. Divergent impact of breast cancer laterality on clinicopathological, angiogenic, and hemostatic profiles: a potential role of tumor localization in future outcomes. J Clin Med. 2020; 9:1708.
Article9. Bao J, Yu KD, Jiang YZ, Shao ZM, Di GH. The effect of laterality and primary tumor site on cancer-specific mortality in breast cancer: a SEER population-based study. PLoS One. 2014; 9:e94815.
Article10. Weiss HA, Devesa SS, Brinton LA. Laterality of breast cancer in the United States. Cancer Causes Control. 1996; 7:539–43.
Article11. Roychoudhuri R, Putcha V, Møller H. Cancer and laterality: a study of the five major paired organs (UK). Cancer Causes Control. 2006; 17:655–62.
Article12. Meleka F, Rarla S. Variation of spread of ovarian malignancy according to site of origin. Gynecol Oncol. 1975; 3:108–13.
Article13. Weiss NS, Silverman DT. Laterality and prognosis in ovarian cancer. Obstet Gynecol. 1977; 49:421–3.14. Komiyama S, Katabuchi H, Mikami M, Nagase S, Okamoto A, Ito K, et al. Japan Society of Gynecologic Oncology guidelines 2015 for the treatment of ovarian cancer including primary peritoneal cancer and fallopian tube cancer. Int J Clin Oncol. 2016; 21:435–46.
Article15. Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009; 114:26–31.
Article16. Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. 2013; 130:493–8.
Article17. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002; 20:1248–59.
Article18. Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecol Oncol. 2003; 90:390–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Everamplification of GER-2/neu Oncogene Detected by Differential PCR and its Prognostic Significance in Advanced Epithelial Ovarian Cancer
- Clinical Significance of HER-2/neu Oncogene Amplification Detected by Differential PCR in Advanced Human Ovarian Cancers
- The Prognostic Significance of Serum CA 125 Levels in Patients with Advanced Serous Epithelial Ovarian Cancer
- Tumor Size as a Prognostic Factor in Gastric Cancer Patient
- Surgical management of ovarian cancer