Obstet Gynecol Sci.
2021 Nov;64(6):473-483. 10.5468/ogs.21154.
Fetal programming: could intrauterin life affect health status in adulthood?
- Affiliations
-
- 1Department of Nutrition and Dietetics, Health Science Faculty, Istanbul Gelisim University, Istanbul, Turkey
- 2Department of Nutrition and Dietetics, Health Science Faculty, Baskent University, Ankara, Turkey
- KMID: 2522471
- DOI: http://doi.org/10.5468/ogs.21154
Abstract
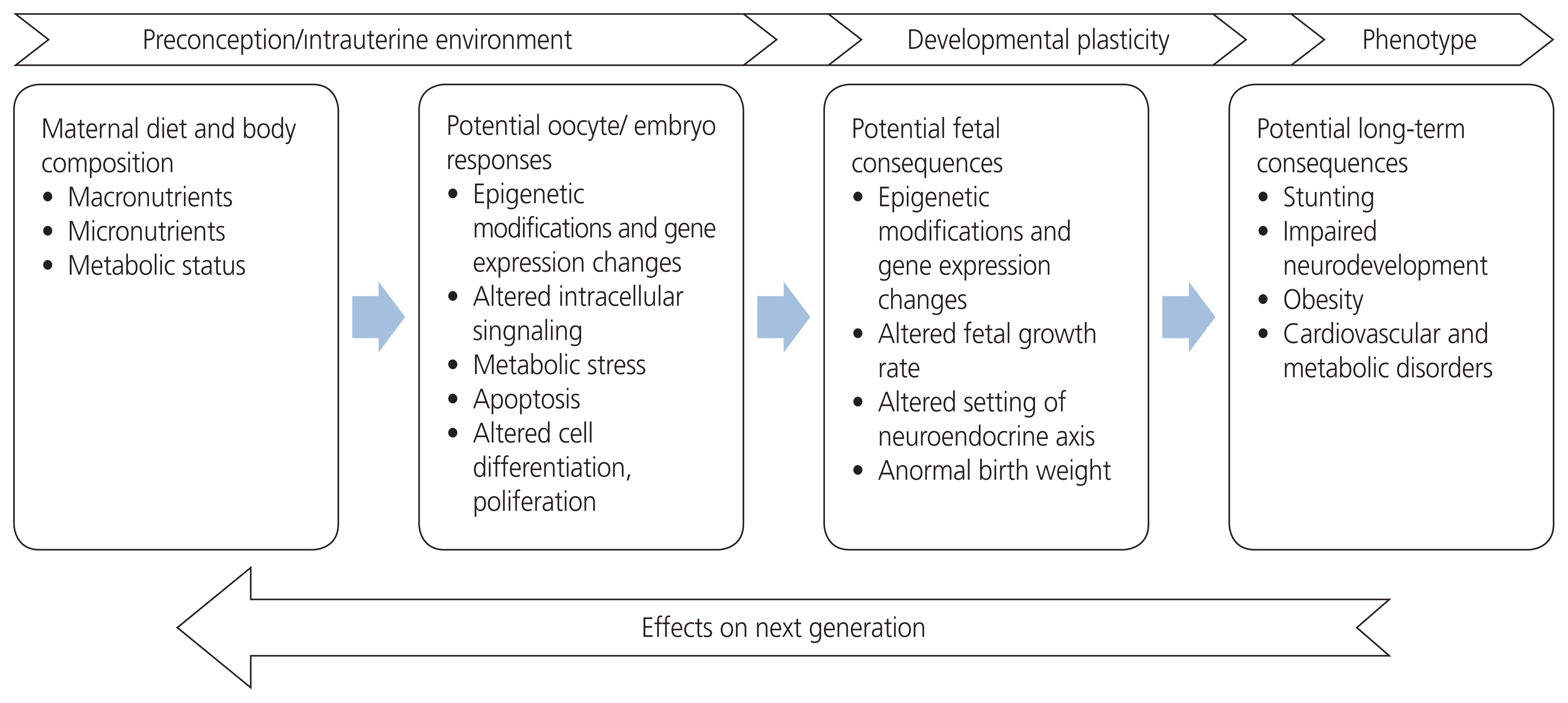
- Intrauterine life is one of the most important periods of life. As the development of the fetus continues, the mechanisms that affect adult health also begin to mature. With the hypothesis denoted “fetal programming,” it is thought that the presence of endocrinological disorders, toxins, infectious agents, the nutritional status of a mother, and nutrients related to placental functionality all have an effect on future life. Therefore, the fetus must adapt to the environment for survival. These adaptations may be involved the redistribution of metabolic, hormonal, or cardiac outputs in an effort to protect the brain, which is one of the important organs, as well as the slowing of growth to meet nutritional requirements. Unlike lifestyle changes or treatments received in adult life, the early developmental period tends to have a lasting effect on the structure and functionality of the body. In this review, fetal programming and the effects of fetal programming are discussed.
Figure
Cited by 1 articles
-
Prenatal maternal alcohol exposure: diagnosis and prevention of fetal alcohol syndrome
Young Min Hur, Jiwon Choi, Sunwha Park, Sarah Soyeon Oh, Young Ju Kim
Obstet Gynecol Sci. 2022;65(5):385-394. doi: 10.5468/ogs.22123.
Reference
-
References
1. Stevenson K, Lillycrop KA, Silver MJ. Fetal programming and epigenetics. Curr Opin Endocr Metab Res. 2020; 13:1–6.
Article2. Marciniak A, Patro-Małysza J, Kimber-Trojnar Ż, Marciniak B, Oleszczuk J, Leszczyńska-Gorzelak B. Fetal programming of the metabolic syndrome. Taiwan J Obstet Gynecol. 2017; 56:133–8.
Article3. Kwon EJ, Kim YJ. What is fetal programming?: a lifetime health is under the control of in utero health. Obstet Gynecol Sci. 2017; 60:506–19.
Article4. Lindsay KL, Buss C, Wadhwa PD, Entringer S. The interplay between nutrition and stress in pregnancy: implications for fetal programming of brain development. biol psychiatry. 2019; 85:135–49.
Article5. Hocher B. More than genes: the advanced fetal programming hypothesis. J Reprod Immunol. 2014; 104–105:8–11.
Article6. Barker DJ. In utero programming of chronic disease. Clin Sci (Lond). 1998; 95:115–28.
Article7. Fitzgerald E, Hor K, Drake AJ. Maternal influences on fetal brain development: the role of nutrition, infection and stress, and the potential for intergenerational consequences. Early Hum Dev. 2020; 150:105190.
Article8. McMullen S, Langley-Evans SC, Gambling L, Lang C, Swali A, McArdle HJ. A common cause for a common phenotype: the gatekeeper hypothesis in fetal programming. Med Hypotheses. 2012; 78:88–94.
Article9. Hocher B, Slowinski T, Stolze T, Pleschka A, Neumayer HH, Halle H. Association of maternal G protein beta3 subunit 825T allele with low birthweight. Lancet. 2000; 355:1241–2.10. Apostolidou S, Abu-Amero S, O’Donoghue K, Frost J, Olafsdottir O, Chavele KM, et al. Elevated placental expression of the imprinted PHLDA2 gene is associated with low birth weight. J Mol Med (Berl). 2007; 85:379–87.
Article11. Yaghootkar H, Freathy RM. Genetic origins of low birth weight. Curr Opin Clin Nutr Metab Care. 2012; 15:258–64.
Article12. Hanson MA, Bardsley A, De-Regil LM, Moore SE, Oken E, Poston L, et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int J Gynaecol Obstet. 2015; 131(Suppl 4):S213–53.
Article13. Moholdt T, Hawley JA. Maternal lifestyle interventions: targeting preconception health. Trends Endocrinol Metab. 2020; 31:561–9.
Article14. Castro-Rodríguez DC, Rodríguez-González GL, Menjivar M, Zambrano E. Maternal interventions to prevent adverse fetal programming outcomes due to maternal malnutrition: evidence in animal models. Placenta. 2020; 102:49–54.
Article15. Elsakr JM, Dunn JC, Tennant K, Zhao SK, Kroeten K, Pasek RC, et al. Maternal Western-style diet affects off-spring islet composition and function in a non-human primate model of maternal over-nutrition. Mol Metab. 2019; 25:73–82.
Article16. Wills AK, Chinchwadkar MC, Joglekar CV, Natekar AS, Yajnik CS, Fall CH, et al. Maternal and paternal height and BMI and patterns of fetal growth: the Pune Maternal Nutrition Study. Early Hum Dev. 2010; 86:535–40.
Article17. Saad AF, Dickerson J, Kechichian TB, Yin H, Gamble P, Salazar A, et al. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am J Obstet Gynecol. 2016; 215:378.e1–6.18. Morgan HL, Aljumah A, Rouillon C, Watkins AJ. Paternal low protein diet and the supplementation of methyl-donors impact fetal growth and placental development in mice. Placenta. 2021; 103:124–33.
Article19. Bischoff AR, Cunha FDS, Dalle Molle R, Maróstica PJC, Silveira PP. Is willingness to exercise programmed in utero? Reviewing sedentary behavior and the benefits of physical activity in intrauterine growth restricted individuals. J Pediatr (Rio J). 2018; 94:582–95.
Article20. Armengaud JB, Yzydorczyk C, Siddeek B, Peyter AC, Simeoni U. Intrauterine growth restriction: clinical consequences on health and disease at adulthood. Reprod Toxicol. 2021; 99:168–76.
Article21. Lakhno IV, Alexander S. Fetal autonomic malfunction as a marker of fetal distress in growth-restricted fetuses: three case reports. Obstet Gynecol Sci. 2019; 62:469–73.
Article22. Gibberd AJ, Simpson JM, McNamara BJ, Eades SJ. Maternal fetal programming of birthweight among Australian Aboriginal infants: a population-based data linkage study. Lancet Glob Health. 2019; 7:e523–32.
Article23. Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000; 108(Suppl 3):545–53.
Article24. Barker DJ. A new model for the origins of chronic disease. Med Health Care Philos. 2001; 4:31–5.25. Eriksson JG. Early growth and coronary heart disease and type 2 diabetes: findings from the Helsinki Birth Cohort Study (HBCS). Am J Clin Nutr. 2011; 94(6 Suppl):1799S–802.
Article26. Vos LE, Oren A, Bots ML, Gorissen WH, Grobbee DE, Uiterwaal CS. Birth size and coronary heart disease risk score in young adulthood. The Atherosclerosis Risk in Young Adults (ARYA) study. Eur J Epidemiol. 2006; 21:33–8.
Article27. Monteiro LJ, Norman JE, Rice GE, Illanes SE. Fetal programming and gestational diabetes mellitus. Placenta. 2016; 48(Suppl 1):S54–60.
Article28. Koos BJ, Gornbein JA. Early pregnancy metabolites predict gestational diabetes mellitus: implications for fetal programming. Am J Obstet Gynecol. 2021; 224:215.e1–7.
Article29. Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008; 300:2886–97.30. Nissen PM, Nebel C, Oksbjerg N, Bertram HC. Metabolomics reveals relationship between plasma inositols and birth weight: possible markers for fetal programming of type 2 diabetes. J Biomed Biotechnol. 2011; 2011:378268.
Article31. Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care. 2010; 33:964–8.
Article32. Hoseini MS, Sheibani S, Sheikhvatan M. The evaluating of pregnancy-associated plasma protein-A with the likelihood of small for gestational age. Obstet Gynecol Sci. 2020; 63:225–30.
Article33. Ramírez R. Fetal programming of adult arterial hypertension: cellular and molecular mechanisms. Colombian Journal of Cardiology. 2013; 20:23–32.34. Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med. 2008; 5(Suppl A):S121–32.
Article35. Bowers K, Liu G, Wang P, Ye T, Tian Z, Liu E, et al. Birth weight, postnatal weight change, and risk for high blood pressure among chinese children. Pediatrics. 2011; 127:e1272–9.
Article36. Franco MC, Casarini DE, Carneiro-Ramos MS, Sawaya AL, Barreto-Chaves ML, Sesso R. Circulating renin-angiotensin system and catecholamines in childhood: is there a role for birthweight? Clin Sci (Lond). 2008; 114:375–80.
Article37. Galbally M, Watson SJ, Lappas M, de Kloet ER, van Rossum E, Wyrwoll C, et al. Fetal programming pathway from maternal mental health to infant cortisol functioning: the role of placental 11β-HSD2 mRNA expression. Psychoneuroendocrinology. 2021; 127:105197.
Article38. Pinto TM, Caldas F, Nogueira-Silva C, Figueiredo B. Maternal depression and anxiety and fetal-neonatal growth. J Pediatr (Rio J). 2017; 93:452–9.
Article39. Jones C, Pearce B, Barrera I, Mummert A. Fetal programming and eating disorder risk. J Theor Biol. 2017; 428:26–33.
Article40. Faa G, Manchia M, Pintus R, Gerosa C, Marcialis MA, Fanos V. Fetal programming of neuropsychiatric disorders. Birth Defects Res C Embryo Today. 2016; 108:207–23.
Article41. Freedman D, Bao Y, Kremen WS, Vinogradov S, McKeague IW, Brown AS. Birth weight and neurocognition in schizophrenia spectrum disorders. Schizophr Bull. 2013; 39:592–600.
Article42. Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 curt richter award paper. Psychoneuroendocrinology. 2015; 62:366–75.
Article43. Allegra A, Giarratana RM, Scola L, Balistreri CR. The close link between the fetal programming imprinting and neurodegeneration in adulthood: the key role of “hemogenic endothelium” programming. Mech Ageing Dev. 2021; 195:111461.
Article44. Kadić AS. Fetal neurology: the role of fetal stress. Donald School J Ultrasound Obstet Gynecol. 2015; 9:30–9.45. Hsu P, Nanan R. Foetal immune programming: hormones, cytokines, microbes and regulatory T cells. J Reprod Immunol. 2014; 104–105:2–7.
Article46. Fisher RE, Steele M, Karrow NA. Fetal programming of the neuroendocrine-immune system and metabolic disease. J Pregnancy. 2012; 2012:792934.
Article47. Wooldridge AL, McMillan M, Kaur M, Giles LC, Marshall HS, Gatford KL. Relationship between birth weight or fetal growth rate and postnatal allergy: a systematic review. J Allergy Clin Immunol. 2019; 144:1703–13.48. Gatford KL, Wooldridge AL, Kind KL, Bischof R, Clifton VL. Pre-birth origins of allergy and asthma. J Reprod Immunol. 2017; 123:88–93.
Article49. Biemann R, Blüher M, Isermann B. Exposure to endocrine-disrupting compounds such as phthalates and bisphenol A is associated with an increased risk for obesity. Best Pract Res Clin Endocrinol Metab. 2021; 35:101546.
Article50. Buck Louis GM, Zhai S, Smarr MM, Grewal J, Zhang C, Grantz KL, et al. Endocrine disruptors and neonatal anthropometry, NICHD Fetal Growth Studies - Singletons. Environ Int. 2018; 119:515–26.
Article51. Banerjee S, Suter MA, Aagaard KM. Interactions between environmental exposures and the microbiome: implications for fetal programming. Curr Opin Endocr Metab Res. 2020; 13:39–48.
Article52. Kiess W, Häussler G, Vogel M. Endocrine-disrupting chemicals and child health. Best Pract Res Clin Endocrinol Metab. 2021; 35:101516.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What is fetal programming?: A lifetime health is under the control of in-utero health
- What is fetal programming?: a lifetime health is under the control of in utero health
- Analysis for the Impact of Adulthood and Childhood Socioeconomic Positions and Intergenerational Social Mobility on Adulthood Health
- Fetal Programming and Adult Disease
- The Maternal Obesity: Adverse Outcomes of Pregnancy and the Offspring